An ongoing dialogue on HIV/AIDS, infectious diseases,
February 15th, 2021
Time to Fix the HIV Testing Algorithm — and Here’s How to Do It
Remember the revised HIV testing algorithm that debuted in 2014? The one that was supposed to solve all our problems?
First, it included a “highly sensitive” screening test that started with a “4th Generation” combination antibody/antigen test. This decreased the window period between acquiring HIV and having a positive test, thanks to the antigen. Great!
(These “generation” analogies sure are common in medicine. Is there a line of inheritance? A royal family? Some black sheep?)
Second, it retired the HIV Western blot as the confirmation test, and substituted a “differentiation assay”, a test that distinguishes between HIV-1 and HIV-2 antibodies. This test is cheaper, faster, more sensitive, and automated, many advantages over the Western blot — may the latter R.I.P, it served us well for many years.
Hooray! All problems solved. A highly accurate diagnostic test in ID just got even better.
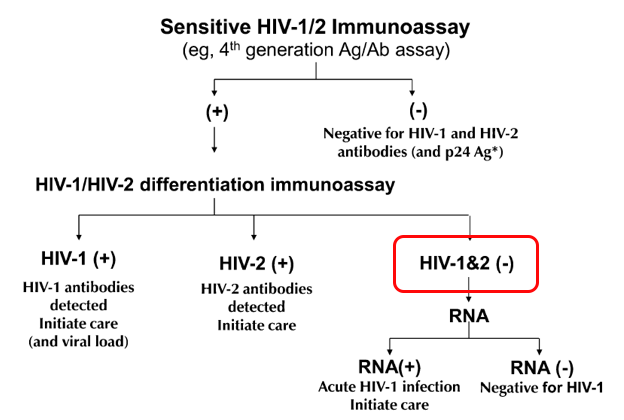
Well … not quite. The algorithm improved, but a major problem remained — what does it mean when a highly sensitive fourth-generation test comes back positive, but the differentiation antibody assay is negative?
In graphic form, this:
These indeterminate results are so frequent that it motivates by far the most common question we ID doctors receive about HIV testing — and was the subject of one of the most popular pre-pandemic posts ever on this site.
The problem is that these results represent two clinical states that are diametrically opposite:
- Acute HIV — a person who recently acquired HIV, and is still in the “window period” before antibody develops. Remember, the differentiation assay is an antibody test. It takes a while to turn positive, typically 2-4 weeks.
- False-positive HIV screen — a person who doesn’t have HIV at all.
In most clinical settings, the second of these (the false-positives) greatly outnumber the acute HIV cases. But we still have to bring back the patient to rule out acute HIV.
Which is a pain, and creates several more “worry days” before there is diagnostic certainty.
Ah, but what if you could just do an HIV RNA assay — a viral load — as the confirmatory test? And potentially not have to bring the patient back in at all?
Such a strategy is now feasible using one of the HIV viral load assays, the Aptima® HIV-1 Quant Dx. It’s the first quantitative viral load test that is also approved for diagnosis of HIV. Off a serum sample sent for HIV screening, the lab can run a qualitative HIV viral load with this assay, with results as follows:
- Non-reactive for HIV-1 RNA, or
- Reactive for HIV-1 RNA
The lab can detect whether the reactive test was < 30, between 30-10 million copies/mL, or >10 million — but the package insert says it won’t report those ranges, since the test isn’t approved this way.
(I should mention here that long-time HIV testing guru Bernie Branson says that says that the assay can report an actual number off of serum, albeit one that is likely lower than we get from plasma. Which makes us wonder whether a plasma sample can be collected for HIV screening, and reflexed to the accurate quantitative viral load — the subject of a collaborative modeling study led by my colleague Dr. Emily Hyle.)
With the HIV RNA as the confirmatory test, the vast majority of reactive HIV screens could quickly be resolved — a non-reactive result would be all but 100% reassurance that the screening test was a false-positive. And a reactive result would mean that person has HIV, and should start ART.
The differentiation assay will still be run if the HIV RNA is negative, as it will determine whether a person is either an HIV controller (has HIV with an undetectable viral load), or has HIV-2. But both are pretty darn rare.
So let’s go back to the confirmatory HIV RNA being reactive. Would this be sufficient information to start treatment, or would clinicians still want to collect a baseline quantitative result, then start ART?
I wondered about this, so here’s a little poll:
Hey #IDtwitter — if the HIV testing algorithm went like this:
1) HIV Ag/Ab screen – Reactive
2) HIV RNA confirmation – Reactive for HIV RNA (range 30-10 million)
Would you still send a quantitative VL before starting ART? Why or why not? @EmilyHyle @Hologic— Paul Sax (@PaulSaxMD) February 13, 2021
A solid majority would still want this quantitative result, even while acknowledging it’s unlikely to change most initial treatment strategies. Here’s one of the best reasons why from Dr. Hana Akselrod:
Patients have mentioned the emotional impact of seeing the VL number come down by orders of magnitude, so that is now part of my motivational interview. Then we segue into U=U.
I agree!
Plus, HIV experts will note that two initial regimens — DTG/3TC and tenofovir/FTC/RPV — also have upper limit viral load thresholds that exclude them as options. (For these two, it’s 500,000 and 100,000, respectively.)
However, from a medical perspective, knowing the precise quantitative viral load usually would fall more into the “nice to know” than “need to know” category.
I’d bring the person back for a resistance genotype (another “nice to know” test) and a plasma quantitative viral load, and start ART (generally bictegravir/FTC/TAF or dolutegravir plus tenofovir/FTC) awaiting the results of these tests.
To summarize, it makes all kinds of sense to use an HIV RNA as the confirmatory test after a reactive HIV Ag/Ab screening test. It will reduce both “worry days” for those who test negative and speed up the time to starting HIV therapy for those who test positive.
Even better? Collect a plasma sample for the HIV screening test, and then use that for a precise quantitative viral load if the screen is reactive.
Now when can we make this change?
And since I haven’t featured a video in a while, how about this one, sent to me by a noted (but now retired) food journalist? How can you resist Weird Stuff in a Can?
We hope you enjoyed this week’s break from relentless 24/7 COVID-19 coverage. Have to mix things up a bit. Thank you for your understanding.



How do you feel about “a rapid start? which ID doc starting ART in the hospital?
I’d suggest the implementation of a “reflex” strategy as we do in HCV diagnosis with great success.
Once a reactive HIV Ag/Ab screening test is identified, the lab searches if this is a new diagosis, and if a HIV VL has recently been performed. If not, HIV RNA is automatically done, with no need to order it.
Completely agree this should be the norm! However, regulatory limitations and laboratory guidelines don’t currently permit this in most testing locations in the USA. You’d be surprised how hard it is to get this implemented!
-Paul
Dr Akselrod is my new hero. She managed to pull off using MI and U=U in the same sentence in my most beloved blog. “These are a few of my favorite things”
This makes too much sense, Paul! Do we think CDC will change their algorithm? That piece may be critical to get laborites to change their processes since many look to those algorithms as gospel. In a setting where close to 10% of new diagnoses in the ED are ‘acutes’ which are also disproportionately young people (the same group that can be hard to get back in for the repeat testing) this would be invaluable. this may be even more important in the COVID era where we may be seeing even a higher proportion of ED diagnoses being acute infections
I had a pregnant patient who tested positive for HIV by the 4th generation Ag/Ab assay but negative for HIV1/HIV2 by 2 different HIV antibody tests and negative for HIV Ag test. HIV viral load was undetectable. Repeated the same tests showed exactly the same results. Both HIV RNA and DNA were negative. Her husband was diagnosed with HIV infection and high HIV viral load on the same day as patient’s 1st visit. What would you do?
Thanks as always for a great post.
A viral load would be preferable. The confirmation testing is a subjective read. Two issues have come up with this in our clinic. Using the standard 4th G screen followed by confirmation both yielding positive result. Subsequent VL has been negative with ultra sensitive assay. Elite controller? Repeat VL remain negative. DNA PCR also negative. Pt refuses ART. Tried old stand-by Western blot and also positive. This remains unresolved. This has occurred more than once. Another example was then negative on repeat confirmation suggesting error in lab with first read.
Insurance, the law in some states and patient piece of mind doesn’t treat a diagnosis of HIV the same way we treat a pos HCV screen with a negative VL unfortunately. Maybe one day. That stigma is a conversation we need to have.
Ultimately I would worry about a Pos 4th G screen that is VL negative and does not have a more specific confirmation of antibody.
The fifth generation HIV detects both the antibody and the antigen too but provides separate results for each analyte. Next-generation Ag assays are discussed in the second link content as well.
https://cvi.asm.org/content/23/4/249
https://jcm.asm.org/content/56/8/e02045-17
*The fifth generation HIV test detects both the antibody and the antigen too but provides separate results for each analyte. Next-generation Ag assays are discussed in the second link content as well.
https://cvi.asm.org/content/23/4/249
https://jcm.asm.org/content/56/8/e02045-17
What about elite controllers? Might this strategy mistakenly call them negative? Same with folks on ART with a nondetectable viral load.
They still get the differentiation assay after the negative/undetectable viral load.
-Paul