An ongoing dialogue on HIV/AIDS, infectious diseases,
March 13th, 2024
CROI 2024 Denver: Really Rapid Review
 One of the most rewarding things about social media in medicine is tapping into the minds of other smart people in your field, especially people you can’t otherwise interact with on a day-to-day basis. When that person is someone like Dr. Sébastien Poulin — a funny and indefatigable ID/HIV doctor from Montreal — it’s especially worthwhile.
One of the most rewarding things about social media in medicine is tapping into the minds of other smart people in your field, especially people you can’t otherwise interact with on a day-to-day basis. When that person is someone like Dr. Sébastien Poulin — a funny and indefatigable ID/HIV doctor from Montreal — it’s especially worthwhile.
Over the past few years, he’s taken the time to scan the entire program of an upcoming ID/HIV conference, selecting his highlights. With the caveat that no one’s highlights will be exactly like anyone else’s, these reviews have nonetheless provided a framework for going to an upcoming meeting that I’ve learned from, valued, and greatly enjoyed.
Well, this year Dr. Poulin could not make the Conference on Retroviruses and Opportunistic Infections (CROI) 2024, which took place in Denver last week. So with a nod to him — get well soon! — here’s a Really Rapid Review™ of some of the studies from this year’s meeting that I found particularly interesting.
It’s the first time CROI has been in Denver since 2006 and, while no one presented a viable strategy for HIV cure or an effective HIV vaccine, we saw plenty of interesting studies. Here’s a sampling, roughly ordered by treatment, complications, and prevention.
After virologic suppression, long-acting injectable cabotegravir and rilpivirine (CAB-RPV) was superior to oral antiretroviral therapy (ART) in those who struggle with ART adherence. So many good things to say about this study — it was randomized, it used economic incentives successfully, it included a population rarely included in clinical trials. And here’s an irony: the net benefits of using CAB-RPV on a per-person basis are likely to be much greater in this study group than in those for whom the treatment is FDA approved, who are already doing well on oral ART. Think about that for a moment.
CAB-RPV injected every 2 months was noninferior to continued oral ART in Africa. Conducted in South Africa, Uganda, and Kenya, the study demonstrated that this treatment would be effective even with infrequent (every 24 months) viral load monitoring and, more remarkably, with a high proportion harboring non-nucleoside reverse transcriptase inhibitor (NNRTI; 10%) and integrase strand transfer inhibitor (INSTI; 8%) mutations on proviral genotypes done on baseline samples. (I still would not recommend this regimen for people with known resistance!) Two of 256 CAB-RPV recipients developed integrase resistance.
Once-weekly lenacapavir and islatravir maintained virologic suppression. The weekly dose of islatravir was 2 mg and did not lower total lymphocyte or CD4 cell counts. This is the first once-weekly oral ART regimen with demonstrated efficacy, with phase 3 studies planned.
Other once-weekly oral agents are in development. These include an integrase inhibitor, an NNRTI, and a nucleoside reverse transcriptase translocation inhibitor (NRTTI).
In a study conducted in Haiti, switching people on a second-line boosted protease inhibitor (PI)-containing regimen to BIC/FTC/TAF was highly effective. Such individuals are known to have high rates of nucleoside reverse-transcriptase inhibitors (NRTI) resistance, so this reinforces the results of the 2SD study, which showed the safety of the boosted PI to second-generation integrase switch in maintaining virologic suppression — even without knowledge of this resistance. Proviral DNA resistance testing is planned. Remarkable that the primary investigators on site in Haiti could conduct this study in a setting with such extreme civil unrest, a heroic task. (Disclosure: I am a co-investigator.)
In an open-label, phase 2 study, daily oral bictegravir plus lenacapavir was compared to a continued complex regimen, with comparable rates of virologic efficacy. While they were given separately in this phase 2 study, a co-formulation of BIC/LEN is currently in phase 3 trials.
Twice-daily BIC/FTC/TAF achieved comparable virologic suppression to TDF/3TC plus DTG twice daily in HIV-related tuberculosis. Impressively, virologic suppression was observed in 97% of participants in both arms. After the presentation, some raised concerns about the unnecessary doubling of the TAF/FTC dose in the BIC/FTC/TAF group, but these agents are very unlikely to cause toxicity even with this increased exposure — and none was observed.
In a large (N=1362) observational study, the effectiveness of CAB-RPV in clinical practice was similar to clinical trials. Confirmed virologic failure occurred in 2% of individuals; a second study (N=278) reported an incidence of 0.9%. In one single-site report, 4% (3 out 75 patients) experienced this negative outcome and are now on PI-based regimens, with a suggestion that irregular injection practices at an independent infusion center may have contributed. Treatment failure with resistance is the most dreaded outcome of this CAB-RPV regimen, so it’s good to have these “real world”* data.
(*Ouch. Some people hate that term. I find it mildly annoying, preferring “in clinical practice”, which my friend Dr. Eric Daar hates. Oh well. But “real world” is so widely used these days it’s hard to avoid — so I succumbed and used it.)
A case series demonstrated the effectiveness of combining long-acting cabotegravir plus lenacapavir. The most commonly cited reason for using cabotegravir without rilpivirine was baseline RPV resistance — which is especially likely in people with long-term HIV and prior treatment failure, and is a major issue globally. From a practical standpoint, cabotegravir alone (without RPV) for HIV treatment is not FDA approved — only for prevention — which means that clinicians must discard the RPV when obtaining it for treatment and used in this fashion.
Ward 86 in San Francisco again reported high rates of viral suppression for people with viremia who were treated with CAB-RPV. Out of 59 such patients followed to week 48, 81% (48/59) remained on LA-CAB/RPV and were virally suppressed; an additional 7 patients were on alternative ART and suppressed. Only 3 of 59 (3%) experienced virologic failure with resistance. These are some of the core data that motivated the change to the IAS-USA treatment guidelines, discussed here previously.
Several studies (abstracts #117-121) with broadly neutralizing antibodies (bNAbs) demonstrated that we’re still far from seeing these agents as part of viable ART strategies. Issues remain the complex, slow, and expensive test for resistance; a high proportion of people with pre-existing resistance once the test is done; intravenous administration (for some bNAbs); and, even if levels achieved are adequate with a susceptible virus, still a higher rate of failure than we see with standard ART. On the flip side, bNAbs may offer the first chance at twice-yearly therapy (with lenacapavir); updated results from that study were presented in a small number of patients.
Two years after a programmatic switch to tenofovir/3TC/dolutegravir (TLD), virologic failure with DTG resistance was observed, but uncommon. Not surprisingly, it was more likely in those who were viremic at switch — individuals who not only struggle with adherence but also are more likely to harbor NRTI resistance mutations.
A separate prospective cohort study showed that people with treatment failure after switching to TLD had low tenofovir diphosphate levels. Levels were particularly low in those with failure on boosted PI regimens prior to the TLD switch, confirming that suboptimal adherence continued to be a problem for people on failing therapy.
Detection of resistance mutations by proviral (“archive”) genotypes over time is highly variable. It’s not that the mutations come and go — it’s that the sampling process may or may not detect them. The take-home message is that this test has a strong predictive value positive for detected mutations, but negatives should be viewed with appropriate caution.
How effective was lenacapavir in patients with no other fully active drugs? In this analysis from the CAPELLA study of highly treatment-experienced patients, 12 participants had zero fully active background agents when treated with lenacapavir. Regardless, 8 of 12 still achieved and maintained virologic suppression. While such a strategy isn’t recommended (always best to use active drugs in combination), it may be necessary in certain individuals under extreme resistance situations. Results remind me of ACTG 364*, when two NRTIs plus efavirenz — another potent but relatively low-resistance barrier drug — still suppressed viremia in 62% despite extensive baseline NRTI resistance.
(*How’s that for a blast from the past?)
In a randomized clinical trial of hepatitis B non-responders, the HepB-CpG vaccine (HEPLISAV-B) was superior to the standard recombinant vaccine in inducing a seroprotective response. Both two and three doses of the vaccine achieved this favorable result. This is the kind of study that should lead to a change in vaccine guidelines.
In patients with positive hepatitis B core antibodies, switching to a two-drug antiretroviral regimen without tenofovir was not associated with transaminase elevation. This applied even to the 118 individuals not on 3TC or FTC; the study mixed those with and without HBSAb. A second study, by contrast, demonstrated hepatitis B reactivation in 1 of 7 patients with isolated anti-core antibody (no surface antibody) when switched to a non-tenofovir and non-3TC/FTC regimen. A take-home message from the various studies to date? Those with isolated anti-HB core antibodies should, if possible, remain on anti-hepatitis B-containing ART; switches to regimens without tenofovir or FTC/3TC should be monitored for hepatitis B reactivation.
Simultaneous initiation of HIV and hepatitis C virus (HCV) treatment was highly effective. The regimens used were BIC/FTC/TAF and SOF/VEL for HIV and HCV, respectively. Out of 128 patients enrolled (52 HIV treatment-naive), all achieved HIV viral suppression, and 98.4% (126/128) had HCV cure by sustained virological response (SVR) 24 assessment. Imagine trying to do something like this in the early ART and interferon for HCV eras — it is extraordinary how far we have come with HIV and HCV treatment!
The final results of the DOXYVAC study were presented — and the meningitis B vaccine just missed demonstrating significant protection against gonorrhea. This randomized trial looked at both doxy PEP and the meningitis B vaccine in a factorial design, and we learned at last year’s CROI that doxycycline postexposure prophylaxis (doxy-PEP) intervention was protective. These updated results showed the incidence of gonorrhea in the vaccine arm was numerically lower than no vaccine (58.3 vs 77.1/100 person-years, respectively), for a hazard ratio of 0.78 (95% CI 0.6-1.01)*. When a result is this close, the most conservative conclusion is that “a small benefit cannot be excluded.” Even if that benefit is real, a better gonorrhea vaccine would be of great use!
(*Time to quote Maxwell Smart.)
In San Francisco, a policy of recommending doxy-PEP to men who have sex with men (MSM) and trans women was strongly associated with a decline in the incidence of chlamydia and syphilis. No significant change was observed for gonorrhea. Additional supportive data on doxy-PEP came from a single clinic site and from the open-label extension of the DOXYPEP study. National guidelines are expected soon; they’re already in draft form.
In a VA-based study, prostate cancer was diagnosed at a later stage in men with HIV versus HIV-negative controls. This finding is of particular interest since prostate cancer has been historically one of the few cancers not observed to have a higher incidence in persons with HIV (PWH), strongly suggesting this result represents a screening gap — which, though controversial as a general tool, is still recommended in higher risk men.
Using data from the REPRIEVE trial, investigators reported that risk-prediction tools underestimated cardiovascular event risk in high-income regions only. The effect was particularly strong in women, who experienced about two and a half times more events than predicted; for Black participants 50% more.
In a prospective single-arm clinical study, semaglutide reduced the amount of liver fat by 31% at week 24. Not surprisingly, weight and glucose control also improved. A second analysis from this study demonstrated a reduction in psoas muscle volume, without impairing physical function. These are two of four studies on glucagon-like peptide 1 (GLP-1) agonists at CROI, and my overall sense is that they’re working the same as in people without HIV — with the caveat that “Ozempic face” might be particularly distressing to people who have baseline lipoatrophy. The slide session started with a terrific review by Dr. Todd Brown.
Switching to a doravirine-containing regimen was associated with weight loss. Although it’s not specifically spelled out in the poster, the bulk of this effect most likely arose from switching the whole regimen to TDF/3TC/DOR, as TDF-based regimens are known to have weight-suppressive effects. Since in a previous clinical trial, there was no change in weight in a previously presented randomized trial of switching from BIC/FTC/TAF to DOR/ISL, it seems unlikely that switching just an INSTI to DOR would lead to weight loss — a question that will be answered by an ongoing clinical trial.
Damage to intestinal enterocytes might explain the weight loss and lipid-lowering effects of tenofovir DF. Twelve men on TDF and twelve on TAF underwent gastroscopy, with biopsies from the proximal and distal duodenum. Those on TDF had more histologic abnormalities (flatter villi and deeper crypts), as well as lower levels of certain nutrients absorbed from the proximal duodenum, and higher levels of serum intestinal fatty acid-binding protein, a marker of enterocyte damage. Both groups had evidence of mitochondrial damage on electron microscopy.
Women who switched to an integrase-based regimen during menopause experienced early accelerated increases in waist circumference and body-mass index. Comparison groups included women with HIV who did not switch regimens, and women without HIV — neither experienced these changes.
In rural Uganda and Kenya, a prevention “package” that offered the options of oral preexposure prophylaxis (PrEP), PEP, and cabotegravir greatly increased the uptake of PrEP over standard of care. The effect was huge — a five-fold increase — and led to a significant reduction in HIV transmissions in those offered the package versus usual care.
In a prospective study of PEP, BIC/FTC/TAF was well tolerated with no seroconversions. Study results support previously published data. Given the low incidence of seroconversion in PEP users, we will never have a comparative clinical trial of different ART strategies that demonstrates one approach is better than another; as such, BIC/FTC/TAF seems like an optimal default choice since it’s simple, well tolerated, and has few drug interactions. Time to include this in PEP guidelines, which are in need of updating?
Three of six children with in utero HIV transmission stopped treatment without viral rebound. All mothers received ART during pregnancy, and the babies started treatment within 2 days of birth. Treatment was stopped at a median of 5 years of age, and the duration of remission was reported as 48, 52, and 64 weeks. Among the 3 children who rebounded, one did not do so until week 80 — hence it’s premature to call these kids cured.
So that’s a wrap! Of course it’s hardly comprehensive, so if I left out your favorite study or studies, have at it in the comments.
Here’s a reminder of the big news out of the first Denver CROI in 2006:
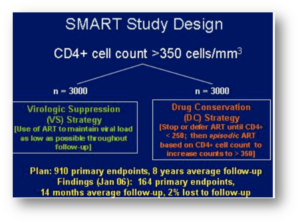
That’s the SMART study of CD4-guided treatment interuption which highlighted the oral presentations. I distinctly remember Dr. Steven Deeks telling me in the hotel lobby, “You’re not surprised at that result, are you? Of course stopping treatment is a bad idea.” As usual, Steve had figured things out long before any of us!
That meeting also featured a trio of drugs destined to change the history of HIV treatment for people with resistant virus — darunavir (TMC-114), etravirine (TMC-125), and raltegravir (MK-0518). In the years to come, many who had never previously achieved viral suppression reached “undetectable” for the first time, and remain so today.


Great summary, Paul!
I spoke with the presenter for the long-active CAB-LEN poster, and he told me that rather than obtaining Cabenuva and discarding the RPV, they were surprisingly able to get Apretude approved in several cases once they explained to the insurer how it would be cost-effective for them.
Thanks for the great summary! And I remember the SMART study — there really was uncertainty about which strategy was better.
Regarding the TB study, you said doubling TAF dose was unnecessary. But I thought that rifampin’s PGB induction meant that it IS necessary to increase the dose, since rifampin lowers tenofovir levels, and the TAF dose is so much lower than TDF.
I’d be comfortable using TAF in people on rifampin even with standard dosing based on this PK study, which showed that intracellular levels of tenofovir DP, even with rifampin, are higher than when TDF is used without rifampin.
https://doi.org/10.1093/jac/dkz068
But your point is a good one, which is that there will be some lowering of tenofovir levels, making the TAF double-dose additionally safe!
– Paul
I wonder how comes that in CROI 2023 the DOXYVAC results showed protection by Bexero of 50%, and now hardly any protection if at all. If you look at the graphs form 2024 CROI, and stop at 12 months it doesn’t look at all similar to the CROI 2023 graph of 12 months follow up.
Any ideas of this differnce?
There was a press release in May 2023 indicating that the full analysis of the meningitis vaccine data from this study could have a different result — and it did!
-Paul