An ongoing dialogue on HIV/AIDS, infectious diseases,
October 17th, 2009
Is Chronic Fatigue Syndrome Another Retroviral Disease?
 Here’s a surprising report in Science:
Here’s a surprising report in Science:
Studying peripheral blood mononuclear cells (PBMCs) from CFS patients, we identified DNA from a human gammaretrovirus, xenotropic murine leukemia virus-related virus (XMRV), in 68 of 101 patients (67%) compared to 8 of 218 (3.7%) healthy controls … These findings raise the possibility that XMRV may be a contributing factor in the pathogenesis of CFS.
I confess, I had never even heard of “xenotropic murine leukemia virus-related virus” (wow that’s a mouthful) before this report, but apparently virologists have been aware of it for some time, due to a possible association with prostate cancer.
The story behind the Whittemore Peterson Institute reporting these findings is almost as interesting as the paper itself. From their web site:
In September of 2004, a group of dedicated citizens and clinicians proposed the concept of a medical institute for the millions of patients in the US suffering from the disorders known as Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), fibromyalgia and other closely related illnesses. They were concerned by the lack of available doctors to understand and serve the growing numbers of patients with these complex chronic illnesses.
According to the coverage of the story in the Times, the Institute got its start with a several million dollar donation from Annette and Harvey Whittemore, whose child has been suffering from CFS for over twenty years. The Times piece goes on to quote Dr. William Reeves from CDC, who sounds quite skeptical about the findings.
“We and others are looking at our own specimens and trying to confirm it,” he said, adding, “If we validate it, great. My expectation is that we will not.”
My take on all this? Despite our being down this road before on CFS — EBV, HHV-6, candida, enterovirus, parainfluenza, Lyme, to list a few putative causes — without much to show for it scientifically, I’m all for having multiple groups working on trying to find the cause of this awful disorder, even if it seems likely that there is more than one cause.
And judging from some of the pained, angry, and frustrated comments posted here, I’m clearly not alone.
October 12th, 2009
AIDS Vaccine: Maybe not Effective After All
Well, that didn’t take long:
Researchers from the U.S. Army and Thailand announced last month they had found the first vaccine that provided some protection against HIV. But a second analysis of the $105 million study, not disclosed publicly, suggests the results may have been a fluke, according to AIDS scientists who have seen it.
In short, two additional analyses (intention-to-treat and per protocol) do not show a statistically significant protective effect. In other words, the study subjects who followed the protocol most closely (per protocol) had less protection from the vaccine strategy.
What the …
But even in the original report, the protective effect didn’t seem all that great — statistically significant, yes, but maybe not clinically significant — so when you add how cumbersome this vaccine is to administer (and probably to manufacture), as well as these new findings, the likelihood of this vaccine strategy making its way to clinical use seems vanishingly small.
So what can we still hope from this study?
At best, some understanding of the correlates of protection. Maybe.
October 5th, 2009
No Baseball Tonight
 “Are you writing another funny blog post because there’s no baseball?” asks my son J.
“Are you writing another funny blog post because there’s no baseball?” asks my son J.
As “funny” is very much in the eye of the beholder, that remains to be seen. But here are a few things on my mind the last few days:
- No data supporting N95 over surgical masks for flu. One huge logistical issue dodged — at least for now. But Consumer Reports, that darling of objective reporting, seems to think they’re just great. Since “minimax” is the operative strategy for infection control, look for this controversy to go on for a bit.
- Speaking of flu, I’ve been hearing for weeks (from a certain someone) how chaotic this H1N1 vaccine situation will be in pediatric practices. And here’s proof.
- LONG (wow) piece on E. coli O157:H7. Not unreasonable, given the potential severity of the illness and the tortuous route hamburgers take en route to our kitchens. (Yuck.) Did I mention before that this is one of the few ID issues that my wife and I take a hard line on? Make those burgers well-done, thank you.
- Hey, did you catch the 10AM agenda in tomorrow’s General Court of the Commonwealth of Massachusetts? Can’t miss that.
- Finally, did a throat infection bring down the mighty Tyrannosaurus rex? Perhaps if the dinosaur doc sent a “hold” tube to the lab, they can make the diagnosis retrospectively.
Good luck Tigers/Twins fans!
September 30th, 2009
The Battle for Colonic Microflora
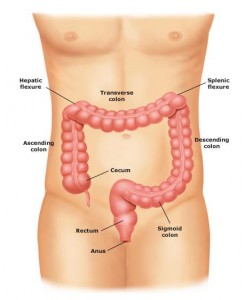 My two favorite newspapers (New York Times and Wall Street Journal — sorry, hometown paper) have just covered opposite ends of a topic on the edges of ID practice — namely, colonic micro-organisms.
My two favorite newspapers (New York Times and Wall Street Journal — sorry, hometown paper) have just covered opposite ends of a topic on the edges of ID practice — namely, colonic micro-organisms.
Too few?
Too many?
Wrong type?
In the Times, a review of the probiotic debate:
Probiotics are live micro-organisms that work by restoring the balance of intestinal bacteria and raising resistance to harmful germs… So what health problems can probiotics really help? After gathering at a Yale workshop to review the available evidence, a panel of 12 experts concluded that there was strong evidence that several probiotic strains could reduce diarrhea, including that associated with antibiotic use. Several studies have also suggested that certain probiotics may be useful for irritable bowel syndrome.
I suspect the “experts” were gathered specifically because they had interest in this topic, and hence may be predisposed to a favorable review of the data. Still, there might be something to it, and I’m crazy about yogurt — full summary of their report here, which was published in 2008.
Far more fringey, however, is the whole “colon cleansing” craze, covered in the WSJ:
The typical American diet of processed foods, pharmaceuticals, stress and lack of exercise is clogging up our lower intestinal tracts, leaving them inflamed and lined with waste—and leaking toxins into the body that cause problems ranging from headaches and chronic fatigue to arthritis and cellulite. All that “stubborn fecal matter” also contributes to bulging bellies and expanding waistlines, cleansing proponents claim.
Eliminating the buildup, either with supplements or laxatives, or by flushing the colon with warm water—a practice known as “hydrotherapy” or “colonics”—can dramatically improve a person’s health and well-being, proponents claim …
Gastroenterologists pooh-pooh [I did not make that up!] many of these claims …
What follows is a very thoughtful review — and a clear debunking — of what seems to be a major form of quackery out there. For example, here are the claims of one such service:
Colon cleansing benefits intestinal tract problems, absorption, bowel disease, constipation, digestive system, parasites, yeast infection. Helps control blood pressure, restores pH balance, restores proper digestion, reduces bad odors. Colon cleansing also clears intestinal blockage, relieves bloating, helps purify blood, kills bad bacteria, viruses. These are just of few of the many benefits one can receive by cleaning the intestinal tract.
The problem, of course, is the scientific data backing up these claims are pretty much non-existent. But hey, it’s just $25 for the “Oxygenated Colon Cleanser,” and $35 for the “Super-Oxygenated.” FREE SHIPPING!
Ultimately, I think one of the gastroenterologists quoted in the WSJ piece really nails it here:
“There is a degree of obsession that goes along with this,” says Dr. Landzberg… Even “natural” laxatives, such as the plants senna and cascara, can harm the bowel, Dr. Landzberg says, adding, “The public has grown increasingly wary of the side effects of pharmaceuticals. I would like to see people bring that same degree of healthy skepticism to ‘natural’ products.”
Wise words indeed.
September 25th, 2009
MRSA in Pets
 As every card-carrying ID specialist knows, hardly anything is more common these days than patients with — and questions about — methcillin-resistant Staph aureus, or MRSA.
As every card-carrying ID specialist knows, hardly anything is more common these days than patients with — and questions about — methcillin-resistant Staph aureus, or MRSA.
And one question I’ve been hearing increasingly these days is “Could I be getting my recurrent infection from Rufus?”
To which the answer is, unfortunately, yes.
(I had a dog named Rufus. No offense intended to people out there actually named Rufus.)
Now along comes this article in the New York Times, which no doubt will stimulate the economy by prompting massive sales of hand-sanitizers, plus a flurry of trips to the vet to have Otto cultured.
(That’s Otto — our cat — in the picture, FYI.)
The article is self-explanatory — pets get MRSA, they can spread it to their owners and back — but did they have to put this caption under the picture of the dog?
INFECTION Don Graff of Belle Mead, N.J., with his English setter, Sunny. The dog contracted MRSA after a spider bite [emphasis added] but was given medication and has improved.
Spider bite! If there’s one thing this medical writer should have figured out in her background research for the piece, it’s that MRSA infections are frequently mistaken for spider bites.
And I’d bet good money that Sunny (the English setter in the Times story) never had one — for which I’m sure he’s quite relieved.
September 24th, 2009
HIV Vaccine Study Shows Promise …
 So says this press release by the US Military HIV Research Program:
So says this press release by the US Military HIV Research Program:
A Phase III clinical trial involving more than 16,000 adult volunteers in Thailand has demonstrated that an investigational HIV vaccine regimen was safe and modestly effective in preventing HIV infection. According to final results released by the trial sponsor, the U.S. Army Surgeon General, the prime boost combination of ALVAC® HIV and AIDSVAX® B/E lowered the rate of HIV infection by 31.2% compared with placebo … In the final analysis, 74 placebo recipients became infected with HIV compared to 51 in the vaccine regimen arm. The efficacy result is statistically significant. The vaccine regimen had no effect on the amount of virus in the blood of volunteers who became HIV-infected during the study.
This is great news, of course; we’ve become so used to hearing gloom and doom about HIV vaccine studies that one can’t help but be excited, despite the relatively low (but statistically significant) rate of protection.
Still, one suspects such a combination vaccine could be logistically difficult to manufacture and administer , especially since one arm of the strategy employs the live-canarypox virus ALVAC vector, and 5 injections were required.
Plus there is the issue of cross-clade protection — the vaccine was designed to protect against the most common strains circulating in Thailand (B and E). While B is quite common in North America and Western Europe, is is far less so in Sub-Saharan Africa, where the HIV epidemic is the most severe.
Nonetheless, if you put this news along with the proven protective effects of male circumcision and HIV treatment — the latter I believe to be greatly underestimated by the medical and non-medical community — things are definitely looking up in the HIV prevention arena.
Further details on the study will be presented at the AIDS Vaccine Conference, October 19-22 in Paris — interestingly a return to the same city where HIV was discovered.
September 16th, 2009
News Flash: The Internet Cannot Replace an Actual Human
 Interested in researching the cause of AIDS? Well go ahead and give NetBase Solutions’ healthBase a try, but don’t expect much in the way of filtering:
Interested in researching the cause of AIDS? Well go ahead and give NetBase Solutions’ healthBase a try, but don’t expect much in the way of filtering:
One of the most unfortunate examples is when you type in a search for “AIDS,” one of the listed causes of the disease is “Jew.” Really. The ridiculousness continues. When you click on Jew, you can see proper “Treatments” for Jews, “Drugs And Medications” for Jews and “Complications” for Jews. Apparently, “alcohol” and “coarse salt” are treatments to get rid of Jews, as is Dr. Pepper!
To be fair, the site seems to have cleaned up its act quite a bit since this report — here’s an example of a search I just did. Most of the results are now much more plausible, but there’s still some wacky stuff there. HIV is the number two cause of AIDS (number two?), and number five is “Abbott” — and I don’t they’re referring to the guy up there in the baseball uniform.
Look, I’m all for using the internet for medical information, and acknowledge I can barely function without it these days. But this kind of advanced search engine takes lots and lots of human oversight, and for now the swarm of medical data out there in web-land can be as misleading as it is vast.
(Hat tip to Graeme M for the link.)
September 12th, 2009
49th ICAAC Starts Today
 Browsing through the program book, I see these topics extensively covered:
Browsing through the program book, I see these topics extensively covered:
- H1N1 and seasonal flu, in all their glory — transmission, pathogenesis, treatment, predictions
- Highly resistant GNR — acinetobacter, carbapenemases, ESBL, etc.
- MRSA — my personal favorite
- C diff — though perhaps a little less this year?
While no one expects ICAAC to be an HIV-focused meeting, usually there are a few important papers presented — last year, for example, NA-ACCORD and raltegravir in treatment-naives debuted at ICAAC.
But unless I’m missing something, I don’t see anything this year on the HIV front of comparable importance. One year blip or a sign of the times?
(FYI, the CDA — that’s the California Dental Association — is also having a meeting here. Their meeting-goers have much better teeth than ID doctors/microbiologists.)
September 4th, 2009
For Suspected H1N1, Get Out the N95 Masks?
 So says the Institute of Medicine’s recommendations for protection of health care workers:
So says the Institute of Medicine’s recommendations for protection of health care workers:
Healthcare workers (including those in non-hospital settings) who are in close contact with individuals with nH1N1 influenza or influenza-like illnesses should use fit-tested N95 respirators … Employers should ensure that the use and fit testing of N95 respirators be conducted in accordance with OSHA regulations.
Every so often — well, more like constantly — my wife (the primary care pediatrician in full-time practice) reminds me what life is like seeing patients outside of a tertiary care, academic medical setting.
Her response to this recommendation to use N95 respirators for evaluation of all “influenza-like illness”?
Amazement, incredulity, bafflement, dismay. Seeing as waiting rooms of pediatricians’ offices in the winter are filled with kids with cough, runny nose, and fever, I can certainly understand her response. If these guidelines were followed literally, everyone in these offices would have to wear such a mask virtually all day — never mind the high cost and short supply of N95s, the logistics of fit-testing everyone, the effect on provider morale, etc.
In short, since following this recommendation is currently impossible, one possible response would be to refer all such patients to hospitals. Bad for everyone.
Let’s hope when the CDC reviews these guidelines, they can provide some more practical (i.e., actually do-able) advice for people in practice.

