An ongoing dialogue on HIV/AIDS, infectious diseases,
December 12th, 2024
Dr. Thomas O’Brien — Expert in Antimicrobial Resistance and Giant in His Field (Literally)
 Dr. Thomas (Tom) O’Brien was born in January 1929, in between the discovery of penicillin (September 1928) and the publication of the findings in a medical journal (May 1929). As noted by his longtime mentee Dr. John Stelling, Tom physically embodied the antibiotic era — quite appropriate for someone best known for his groundbreaking work in antimicrobial resistance. He died this week at the age of 95.
Dr. Thomas (Tom) O’Brien was born in January 1929, in between the discovery of penicillin (September 1928) and the publication of the findings in a medical journal (May 1929). As noted by his longtime mentee Dr. John Stelling, Tom physically embodied the antibiotic era — quite appropriate for someone best known for his groundbreaking work in antimicrobial resistance. He died this week at the age of 95.
A longtime faculty member here at Harvard Medical School and Brigham and Women’s Hospital, Tom inspired a crowd of people like me who knew him as a wonderful colleague and mentor. Regularly fielding questions about tricky drug-resistant bugs, he also radiated enthusiasm about the entire field of Infectious Diseases in a way that was never boastful or showy.
I can easily still picture his smiling face towering over the rest of us during weekly plate rounds in the microbiology laboratory, taking such pleasure in the minutiae of a surprising isolate or advance in diagnostics. “Look at this plate of VRE,” he once memorably said. “It grows better with vancomycin. How about that?”, followed by a quick chuckle of amazement at the smarts of microbes.
Tom also was the person whom I first heard describing the framework for thinking about patient care, leading to the The Four States of Clinical Medicine and the perfect two-by-two table. Just wonderful — I think about this all the time.
Here are a couple of personal anecdotes, if I may:
Way back when I was just starting out as a faculty member, still in my early 30s, I received a consultation on a patient with slow-to-resolve severe cellulitis. (Some details in the case have been changed for confidentiality.)
She had chronic leg edema, so was vulnerable to these infections, and this was the second hospitalization for this problem. She also was a high-powered professional here in Boston, a leader in her company; when I first met her, the most striking thing was the pile of impressive-looking documents and thick reports on her hospital tray table as she continued to work despite the infection.
(This was before we had laptop computers or the internet. I told you it was “way back when.”)
I introduced myself as the ID attending, and she shot me a glance over her reading glasses that could not have expressed disappointment more clearly. You? the look communicated, all but telling me, I am not optimistic that you can help me — you’re too young.
After completing the history and physical exam, I told her that the antibiotics the medical team had chosen were fine, but that sometimes these infections — which are usually caused by strep, the same or similar bacteria that cause strep throat — can take a long time to improve. Elevating the leg as much as possible could help speed the process.
Well! This was clearly not what she wanted to hear. Sighing with frustration, she told me this was unacceptable and requested a second opinion — and here’s the kicker: “… from someone older, more experienced.”
Tom O’Brien to the rescue! I saw Tom in the microbiology laboratory and explained the situation. Smiling — he was always smiling — he generously said, “Let’s go up there together and speak with her. My gray hair might reassure her.”
(He did have impressive hair. Family trait, I guess.)
To say that Tom’s kind, gentle manner helped diffuse a tense encounter barely begins to describe how calming his presence in the room was. Lowering himself down from his 6-foot, 5-inch (estimate) vantage point, and sitting by her bed, he gently gave her his thoughts in friendly, easy-to-understand terms, reinforcing what I said without in any way diminishing or ignoring her concerns. It was a master class in doctor-patient communication, and I’m grateful to this day for it.
The rest of her hospitalization she was pleasant — and patient — both with me and the slow improvement. But improve she did!
Here’s a second anecdote: My wife and I were at a divisional holiday party a year or so after the birth of our second child. Struggling big-time with two careers and two little kids, we asked Tom and his impressive wife Ruth (a corporate lawyer) how they managed — especially since they had six (!) children.
“Oh, you do your best,” he said. “Just make sure you show up for their big events. And there’s no shame in getting help.” He then proceeded to tell us that they had an account with a local cab company (this was before Uber), and that their kids happily shuttled from activity to activity using taxis while their parents were at work.
It’s not that opening an account with a cab company solved all our problems. It was his acknowledgment, in such a friendly, unpretentious way, that this parenting thing was tough but that you get by, somehow. If they could do it with six kids, certainly we could do it with two. Something about his calm and cheerful demeanor in the face of this challenge made everything seem better — an approach Tom had about many problems.
(Someone told me that he sometimes said he raised five, not six children — “the sixth one we just threw in there with the rest, and let them take care of raising number six.”)
Dr. Thomas O’Brien, you will be missed! And though I started this piece writing that he is “best known for his groundbreaking work in antimicrobial resistance,” some would argue that perhaps another fact about Tom should take that top spot. I’m speaking, of course, about his son.
Conan, my condolences to you, your mother, Ruth, and your whole family. He was a great one.
(Edit: I learned after posting this that Conan’s mother Ruth, age 92, died 3 days after her husband Tom.)
December 8th, 2024
Who’s Going to Get Lenacapavir for HIV Prevention?
 At the International AIDS Conference this past summer, Dr. Linda-Gail Bekker brought down the house presenting the results of the PURPOSE 1 trial of twice-yearly injectable lenacapavir for prevention of HIV in women. The results — zero infections out of over 2000 participants — demonstrated clear superiority over oral PrEP with TDF/FTC. The study simultaneously appeared in the New England Journal of Medicine; always wonderful timing when that happens.
At the International AIDS Conference this past summer, Dr. Linda-Gail Bekker brought down the house presenting the results of the PURPOSE 1 trial of twice-yearly injectable lenacapavir for prevention of HIV in women. The results — zero infections out of over 2000 participants — demonstrated clear superiority over oral PrEP with TDF/FTC. The study simultaneously appeared in the New England Journal of Medicine; always wonderful timing when that happens.
Now, with a similar study design (without the TAF/FTC arm), we have the results of PURPOSE 2, which tested lenacapavir for HIV prevention in men who have sex with men and people who are gender diverse. Again, the injectable approach significantly beat out oral PrEP, with only two infections occurring among nearly 2000 lenacapavir recipients, around a 10-fold lower rate than in the TDF/FTC arm.
These studies highlight one of the great joys of HIV medicine, in that occasionally we get a study result that’s so dramatic it makes everyone in the field wake up and go wow. To jog your memory, here are five previous 5 wow-moments, at least in my opinion:
- Zidovudine during pregnancy markedly reduces mother-to-child transmission.
- Triple therapy with protease-inhibitor/dual NRTI-based ART improves survival — a lot.
- Integrase inhibitor–based salvage treatment gives nearly everyone a chance at viral suppression, even those with multi-drug resistance.
- Pre-exposure prophylaxis works for prevention of HIV in people at high risk for HIV.
- Suppressive HIV treatment is 100% effective for prevention of HIV transmission.
After each of these studies appeared, everything changed — and changed fast. Guideline writers scrambled to update their recommendations, and HIV treaters, clinical researchers, and community activists strongly advocated for changes to the standard of care.
Will the spectacular results of the PURPOSE 1 and 2 trials meet with the same rapid change in guidelines and rapid adoption? There are reasons to think they will, and reasons to think they won’t.
On the favorable side are, obviously, the incredibly good results — so good that many media reports incorrectly cited these twice-yearly injections as a “vaccine”. Hey, quick fact check, it’s not a vaccine!
(Though parenthetically, one does speculate that something this effective will make HIV vaccine research even harder than it is already. How can one demonstrate better protection with a vaccine than seen in the PURPOSE studies?)
The contrarians will cite the current high cost of lenacapavir as treatment, especially compared to generic TDF/FTC, which CostPlus drugs now lists at $32 for a 3-month supply, and many government-sponsored programs will pay for entirely.
Plus, we have the cabotegravir experience in the “real world”, a sobering reminder that efficacy does not equal effectiveness — or at least not if the breakthrough treatment isn’t put into practice. Remember, cabotegravir was also significantly more effective than TDF/FTC in two blinded clinical trials, demonstrated 100% efficacy in women (if one excludes the participants with HIV at baseline), and has been FDA approved for HIV prevention since December 2021.
And our use of cabotegravir so far in the USA? Based on a recently published CDC report of national prescription trends for PrEP, it was around 3% of those on PrEP in 2023; someone who works on PrEP implementation research told me that it’s only a bit higher today. And its adoption in the parts of the world with the highest HIV incidence is, sadly, essentially zero.
FDA approval of lenacapavir for PrEP is pretty much a sure thing, and expected some time in 2025. Who will get it for HIV prevention promises to be one of the more fascinating stories in HIV medicine over the next couple of years. Stay tuned.
November 27th, 2024
Some ID Things to Be Grateful for This Holiday Season — 2024 Edition

Not a fan.
The calendar says it’s nearly the fourth Thursday of November, so here in the United States, the Thanksgiving holiday is upon us. It’s a day when we gather with family and friends to express thanks, to eat plenty (usually too much), to watch a bunch of spectacular athletes bash themselves to smithereens in the name of sport, and to wonder why anyone would eat sweet potatoes with marshmallows.
(Must be the same people who eat candy corn during Halloween. Yuck on both accounts. And no, I cannot be convinced otherwise.)
It’s also time for me to take stock of our ID world, citing some things in our field that I’m grateful for — an annual tradition on this site. Off we go:
Twice-yearly lenacapavir was really effective for HIV prevention. How could this not garner first mention? While it awaits FDA approval for this indication, and making it broadly available will be a critically important challenge, this should a major advance in pre-exposure prophylaxis (PrEP) for HIV.
We’re about to get a bunch of new drugs for treatment of urinary tract infections. The FDA already approved sulopenem etzadroxil with probenecid and pivmecillinam, and they granted gepotidacin priority review (approval expected by March, 2025). Since UTIs are increasingly caused by resistant organisms, I count this as progress. Naysayers will say, “But what about resistance, side effects, cost, and access?”, so allow me to cite the ID doc’s consistent ambivalence about new antimicrobial agents.

But since this is a gratitude post, I’ll just go with the glass half full here — and hope I can figure out how to say sulopenem etzadroxil with probenecid without using the trade name (it might be impossible).
Three additional studies confirmed the remarkably high resistance barriers of dolutegravir and bictegravir. The results of D2EFT (pronounced “DEFT”), VISEND, and a study from the GHESKIO treatment center in Haiti all gave the same message — that these two integrase inhibitors could be used with tenofovir/3TC or FTC and still achieve or maintain viral suppression, regardless of the degree of baseline NRTI resistance. Amazing! Great to have confirmatory data in support of the prior NADIA and 2SD studies.
Seven days of antibiotics was noninferior to fourteen days in the treatment of bloodstream infections. What a great clinical trial — ask an important and common clinical question, set up the primary and secondary endpoints to be of substantial interest, and then power it appropriately to answer the question. The results provide some solid evidence for The Rules, at least if Staph aureus and endocarditis aren’t in the picture. Why can’t we have more clinical trials like this? Can’t wait to hear what they find in secondary analyses, and in their follow-up BALANCE+ studies.
Metagenomic sequencing and other advanced molecular techniques are slowly making their way into clinical practice. I’d bet good money that if I asked 100 practicing ID docs whether they’ve had at least one case where one of these tests provided a solid, practice-changing diagnosis — rapidly and without an invasive procedure — more than 90% would raise their hand. Maybe 99%.
Rwanda appears to have contained the Marburg virus outbreak. In doing so, the country provided a model for pathogen response, involving enhanced testing, rapid initiation of isolation policies, and scaling up supportive care. If there are no further cases by December 21, the outbreak will be declared over.
The DHHS HIV treatment guidelines removed abacavir from its list of recommended initial treatment regimens. If you combine abacavir’s deficiencies (hypersensitivity, cardiovascular risk, lack of hepatitis B activity, pill size) with the remarkably high effectiveness of dolutegravir/lamivudine and the renal and bone safety of tenofovir alafenamide, there’s hardly any reason to use abacavir anymore — you really have to do some mental acrobatics to come up with a compelling indication. Nonetheless, I suspect HLA-B*5701 will live rent-free in our brains forever.
Non-ID gratitude section:
eBikes are now widely available, in many different styles and at a broad range of price points*. If you haven’t tried one yet, what are you waiting for? They are miraculous machines, allowing you to ride unthinkable distances and zoom up steep hills, so therefore can replace cars on many routes. I already have over a thousand miles on mine and have had it only around a year. (*What’s the difference between a “price” and a “price point”. Gosh if I know.)
Andrea Petkovic is a very fine writer. Two things I’m obsessed about are tennis and great writing. How exciting it’s been, therefore, to discover a professional tennis player (now retired) who provides both? Her writing alternates topics breezy and serious, the tone is deft, human, and humorous, and every week there’s a new selection — the most recent example a beautiful rumination on the retirement of Rafael Nadal. The strength of self-published newsletters comes to life in examples like this.
I discovered at least one thing that Microsoft Teams does better than Zoom. Perhaps unsurprisingly, it’s sharing a Powerpoint presentation. Here’s the brief summary: if you share the file rather than sharing your screen, it gives you a great look at your upcoming slides and other features. It’s the “Presenter View” without having to do anything. Transformative!
Jim Gaffigan is skinny now, and even better, he has a new comedy special. He naturally starts out by explaining how he got his new sleeker physique (this will be no surprise), and the responses he’s received from others — all A+ comedy material from this brilliant and prolific comedian. But he quickly veers off into much broader territory, in particular parenting (he has 5 kids) and, in one particularly funny bit, technology.
Take it away, Jim!
Hey, I’ve been doing this post for years now and expect that I’ve missed one of your favorites. What are you grateful for this year?
November 18th, 2024
Marking a Social Media Mass Migration — Until the Next One
 Periodically my wife and I will have a bunch of trainees (medical students, or residents, or ID fellows, or a mix) over to dinner. Seated around a big table, with no time-crunch of rounds, pagers, or EPIC orders, we can all get to know one another in this more relaxed setting.
Periodically my wife and I will have a bunch of trainees (medical students, or residents, or ID fellows, or a mix) over to dinner. Seated around a big table, with no time-crunch of rounds, pagers, or EPIC orders, we can all get to know one another in this more relaxed setting.
Plus, they get free food, never a bad thing in the years before you start having a real salary.
It helps at these events to have a few icebreaker questions ready in case the guests are nervous, or just because the answers to the questions are so darn interesting. One of our favorites is the classic, What do you love to hate, and hate to love?
To clarify the question, for the purposes of this post, love to hate means something you feel with such a passion that you’ll go on and on about how much you hate it. For years, my answer was readily at hand — football, the American version. I bore people at social events on this topic, despite being in the minority, and once even wrote an opinion piece making my argument.
But for the past 2 years, my response has changed. The thing I now love to hate is what a certain billionaire has done with the site formerly known as Twitter.
(Sorry, I can’t refer to it by the new name. That name is so unimaginative, so eye-rollingly juvenile that it hurts even to write it. Truly.)
As I’ve noted before, the appeal of the original Twitter snuck up on me, with my participation essentially zero for years after I signed up. Then I discovered it was the easiest way to keep up on the latest advances in our field — infectious diseases specifically and medicine in general.
During the pandemic, it became all but indispensable, as changes to the best treatment and prevention strategies came so quickly that I felt I had no way of staying current without it. Experts from around the world — people I otherwise never would have known about or met — enhanced its value, weighing in on strengths and weaknesses of new studies.
I supplemented this informational use of Twitter with several other useful tasks. I could boost the accomplishments of others, promote my own work, and (no small thing) indulge in several miscellaneous hobbies (baseball, tennis, cute dog videos).
The fact that I’d figured out a way to tweak the settings so that attackers couldn’t view or respond to my posts — and strictly followed Peter Sagal’s rules — made some of the more widely publicized limitations of Twitter something I was able to avoid. It was like having an effective social media vaccine.
So what happened? Since October 2022 — note date — my experience on the site has steadily deteriorated. The effectiveness of that vaccine has faded. So what happened?
- Curation now fails. The algorithm either stinks, or most of the people in my field stopped posting interesting things, or both — hence I’m rarely seeing good ID content. Lots of what I get in my feed is irrelevant junk. Pointless ads. Spam.
- Limits on the blocking function. Why on earth would they remove this key safety feature?
- Limits on displaying graphics generated from certain URLs, or limits on those URLs entirely. Surprise, surprise, they might be competitors, or sites that someone (guess who) doesn’t like.
- A certain person’s posts pop up repeatedly, even though I don’t follow him. Hint: He owns the place.
- Bots. Who’s real and who isn’t? Very hard to tell.
- Payment is regularly requested to boost influence. Wasn’t “flattening hierarchies” one of the proposed benefits of Twitter, our “digital town hall”?
- The stupid new name. See above.
That’s plenty. Thanks to the prescient departure from Twitter of my colleague Dr. John Ross years ago, and the energetic assembly of ID “starter packs” by Dr. Ilan Schwartz, I’m joining hundreds of ID docs and millions of others who went over to Bluesky in the past couple of weeks — here I am. That’s a new background photo of Louie, featuring one of the silly plates my wife discovered in a remainder bin.
And wow, this has been a fast switchover, nicely documented both by the mainstream media and more specifically for us ID docs, by Dr. Ken Koon Wong. (That’s his figure on ID engagement at the top of this post, used with his permission.) If you’ve been active as a poster or just lurker on Twitter, I’d encourage you to give Bluesky a try. Here’s Ken’s excellent guide on how to get started.
Let’s see what happens over time. I have no illusions about the permanence of this move, as nicely articulated in a recent piece in The Atlantic. Ignored or abandoned social media sites (MySpace, Friendster, Mastodon, et al.) lay at the bottom of digital scrap heaps like unused USB drives in your desk drawer from a decade ago. Plus, I have no idea how the owners of Bluesky will find a way to support it financially.
Note that the above list of reasons to leave Twitter left off its political associations. Honestly, that is the one thing that I’ll probably miss the most about leaving the site — the exposure to perspectives different from my own. Bluesky has already been accused of being an “echo chamber,” a notorious limitation of social media in general, most notoriously Facebook — of course your “friends” agree with most of your views!
Other limitations of Bluesky are more technical than systemic. You can’t easily bookmark posts. There’s no app optimized for tablets. No polling function. Their servers sometimes struggle with the massive influx of new users. And I have no idea how they plan to make money on this thing, which ultimately I guess will determine its longevity.
Importantly, I haven’t found most of the cute dog feeds yet. But I’m hopeful since WeRateDogs has made the move!
This is Lily. She's been accused of drinking her mom's slushie. Will not be taking any questions at this time. 14/10
I trust you, Lily.
So this is where I’ll be in the social media universe, at least for the time being. Looking forward to more of that great learning I had from Twitter in the pre-2022 era — Bluesky can’t be far off if Dr. Tony Breu has already posted.
And if you’re wondering what I hate to love (the other part of the two-part question), it’s potato chips. Irresistible, but not salubrious.
And it looks like I’m not the only one.
Today in relatable science: Gulls making a mysterious daily trip that turned out to be to a potato chip factory
— Brooke Jarvis (@brookejarvis.bsky.social) 2024-11-15T20:15:15.323Z
November 12th, 2024
Musings About a Bruising and an ID Link-o-Rama

An ID Link-o-Rama — get it?
We’ll get to the ID links in a moment, but first, allow me to share a few words about the election, which strangely feels like a million years ago.
(It was a week. Time is strange.)
Instead of rehashing what happened and what’s to come, here’s what I’m offering: some feelings from one specialist in infectious diseases — me.
I’ll start by saying that long-time readers can probably surmise that the winner on November 5 wasn’t the candidate I voted for. I know, shocking.
Nor would you be surprised to hear that most of my ID colleagues voted the same way I did. Not all of them, of course — our political leanings may tilt heavily one way, but they’re not unanimous.
Still, as the results of the election rolled in and it became evident that they would yield an incontestable win for the Red Team, it occurred to me (as it did in 2016) that our pervasive opinion puts us outside the norm. That’s usually okay — why be mainstream all the time? I think we ID doctors wear our distinctiveness with pride, if not with fancy clothes.
But the post-election feeling among most of my colleagues (and me) was one of disbelief — how could someone vote for that guy? It also made me feel isolated, and sad.
This is especially the case since this country of ours happens to be one that I have deep affection for, warts and all.
Truth be told, even with the election of a man who claimed that recent immigrants are eating dogs and cats, and who once suggested that injecting a disinfectant would treat a novel coronavirus, there’s no place else I’d rather live. Here’s one big reason why: It’s that I have the freedom — the right — to voice a critical opinion of our president-elect, and that I proudly voted with the 72 million and not the 75 million voters. Let’s not take that freedom for granted, and continue to defend it.
On to the ID links, a baker’s dozen to prepare us for Thanksgiving pies, just a bit over 2 weeks from now.
Ensitrelvir prevented the development of symptomatic COVID-19 among household contacts. These are potentially exciting results, especially since Paxlovid did not work in a similar prevention study, and whatever monoclonal antibody du jour we’re using seems doomed to fail eventually as variants emerge. Ensitrelvir is a SARS-CoV-2 protease inhibitor approved for use in Japan and Singapore; I’m very hopeful we’ll have access to it here as well.
7% (8 out of 115) of dairy workers had serologic evidence of highly pathogenic avian influenza A(H5N1). That seems like a lot, doesn’t it? Whether H5N1 will eventually yield an increase in influenza case numbers and/or per-case severity during this or future flu seasons remains unknown, but most definitely deserves close watching.
A large collaborative group of ID pharmacists and doctors published WikiGuidelines for prevention and treatment of urinary tract infections. What a sensational resource of data on diagnosis, treatment, and prevention! Really great. But somewhere along the way I lost the meaning of the term “Wiki” as it applies to mega-projects like this — what does it mean?
Once again, macrolide resistance was strongly predictive of poor outcomes in pulmonary disease due to M. avium complex. Those with MICs ≥32 µg/mL had an odds ratio of 0.25 for achieving microbiological cure.
Omadacycline treatment of M. abscessus led to a faster resolution of symptoms and better microbiologic clearance than placebo. It’s a small, phase 2 study, but it would be huge if eventually omadacycline gets FDA approval for this difficult-to-treat infection.
Related, here is a “State-of-the-Art” review of nontuberculous mycobacterial pulmonary disease. Written by top experts in the field, it covers all the main topics in a practical, informative way. Highly recommended, as I do all the State-of-the-Art Reviews in Clinical Infectious Diseases!
Cases of pneumonia due to mycoplasma have increased substantially in children and young adults. I first heard of this through my most reliable (and readily available!) source of community infectious outbreaks — my primary care pediatrician wife, who says recently “everyone” seems to have it. Helpful clues (per her) are failure to respond to beta-lactams and those weird mycoplasma-related rashes.
Immunity following yellow fever vaccination appears to be durable. Breakthrough cases occur but very rarely. This paper supports the recommendation that a one-time shot for travelers need not be repeated, so very good news.
In its first year of availability, the RSV vaccine reduced the risk of hospitalization by 80% among adults over 60. It was also effective in immunocompromised hosts. It remains unclear whether these vaccines will need to be repeated; for now. there is no recommendation to do so.
According to this preprint study, by the end of 2023, 99.9% of the U.S. population had immunologic exposure to SARS-CoV-2 via either infection or vaccination or both. Previous infection was also nearly universal, at 99.4%. It’s in this immunologic milieu that current antivirals for COVID-19 must make their impact — a tall order, but I believe still achievable with appropriate symptom-based endpoints.
In data collected from two academic medical centers over 5 years, uptake of anal cancer screening for men who have sex with men who have HIV was very poor. Additionally, cytology performed badly in both sensitivity and specificity, raising questions about whether we should be doing it all (versus referring high-risk individuals directly for high-resolution anoscopy). Am I the only one who thinks that the guidelines issued for anal cancer screening are impractical to implement and not evidenced-based?
Using a hospital-based database, investigators found that 41% of patients with babesiosis had coinfection with Borrelia burgdorferi, the etiologic agent of Lyme disease. I knew it was high, but didn’t think it would be that high — should probably prompt empiric doxycycline in everyone newly diagnosed with babesia. Wow. By contrast, ehrlichiosis and anaplasmosis occurred in only 3.7% and 0.3%.
There is very little good evidence on the optimal use of long-term suppressive antibiotics therapy, prompting this sensible review. The four main categories they cover are prosthetic joint infections, hardware infections of bone not involving joints, vascular graft infections, and cardiac implantable electronic devices. The authors also offer advice about monitoring and situations to consider antibiotic cessation.
Ok, now a few bonus non-ID links — just one involving politics, I promise!
The New Yorker just reposted Claire Keegan’s exquisite short story “Foster”. It was later published as a novella, and made into a movie called The Quiet Girl. Some think the book is better than the movie, others the reverse. Regardless, I can’t recommend either of them strongly enough. The movie for another great book of hers, Small Things Like These, has just been released. Anyone see it yet?
For a laugh-out-loud read that will resonate strongly with those charged with writing letters of recommendation, give Dear Committee Members by Julie Schumacher a try. Giving new meaning to the term epistolary novel, the book captures the life of a middle-aged academic who’s clearly past his prime, but still has quite the way with words. I heard about this book from my friend (name dropping, sorry) Andy Borowitz, so thank you, Andy!
Speaking of, Andy Borowitz thinks the President-elect is in for a world of trouble. Love this quote: “As for Trump’s war on inflation, the skyrocketing prices caused by his proposed tariffs will make Americans nostalgic for pandemic-era price-gouging on Charmin.”
The online piano instructional company Pianote has some astounding videos on YouTube. The most remarkable are those where they play a song for the first time to a gifted musician, to see what they can do with it on the spot. See the video below for a remarkable example.
Makes a person optimistic about the capabilities of the human species, doesn’t it?
October 30th, 2024
The Riveting Conclusion of How PCP Became PJP
Before I get back to the saga of Brave New Name — How PCP Became PJP and Why It Matters, allow me to share that I had some trepidation about publishing this thing.
A deep dive down a hole with very high-risk for tularemia exposure (see what I did there?), it veered off topic more than half-baked Tesla Robotaxi loses the roadway during a driving snowstorm. Worried, I tested a draft of Part 1 out on a regular reader I trusted very much. (Ok, ok, my Mom.) She confessed she was “skimming by the end”, which made me concerned I’d gone too far.
But when I got this after posting Part 1, I knew the piece had hit its target:
Kind email from Henry, shared with his permission.
Dr. Henry Masur! The Pneumocystis Expert Extraordinaire is “very pleased”! Amazing!
Now, back to our story.
(Read this next sentence like a voiceover starting the next episode in your favorite series … )
Previously, on Brave New Name — How PCP Became PJP and Why It Matters, we learned that the cause of the most famous HIV-related opportunistic infection — Pneumocystis carinii — was actually a rat fungus, and not a human pathogen after all. The name Pneumocystis carinii pneumonia, and its abbreviation PCP, were both in jeopardy.
If we take the perspective of the scientists making the discoveries about the genetic basis of the organism, this was not a time for sentimentality — it was a time for facts. Pneumocystis carinii was not the bug infecting humans, and that error deserved correction.
To solve this problem, a group of motivated researchers gathered in 1999 at a meeting to settle the issue once and for all. (That meeting must have been a banger.) In a bold move strongly supported by the molecular evidence, they renamed the human pneumocystis Pneumocystis jiroveci (more details here without a paywall) in honor of the the Czech parasitologist Dr. Otto Jirovec who first described the infection in humans.
(Ostensibly the first. Read on.)
Shock waves resonated through the ID and microbiology community. I remember walking down the street in Back Bay, Boston, one July evening in 1999, enjoying some warm summer breezes, and suddenly I heard a loud screams in the distance. What had happened?
Pedro Martinez had struck out 5 of the 6 betters he faced in baseball’s All Star game at Fenway Park. It had nothing to do with this Pneumocystis name change. Still, this novel last name (jiroveci?) of human Pneumocystis caused an multifactorial crisis of international proportions. Among the problems, here are a quick half-dozen:
1. No one knew how to pronounce it. This confusion continues today.
Hey Julien! Thanks for taking the time to make this video. Good try (and lovely music!), but a brief search would have yielded the correct pronunciation, which is “yee row vet zee”. Say it again with me — yee row VET zee.
Of course now that you’ve learned it, we’ll share that some think it should be “VET-chee”, not “VET zee”. But this is hardly the only issue.
2. The original spelling ended with one “i,” when in fact it should be two — jirovecii, not jiroveci. The error arose out of a conflict between standards set by the International Code of Zoological Nomenclature (one “i” for a parasite) versus the International Code of Botanical Nomenclature (two “i’s” for a fungus). And yes, someone published a letter on this topic. (Wow, talk about padding your CV.)
Now say it again with me — yee row VET zee aye. Or, if you prefer, yee row VET chee aye.
Brief aside: (Editor: Yeah sure.) How many International Codes of Nomenclature are there? Where do the committees making the decisions meet? Do they get swag (water bottles, T-shirts, fleeces) at their meetings? If so, I’d like someone to send me a backpack with an “International Code of Canine Nomenclature” logo, which I’m sure has a cute pup on it. Thank you.
3. Not everyone agreed the name should be changed. Dr. Walter T. Hughes (remember him? he’s the “Famed Pneumocystis Guru” from Part 1, the guy with the Cushingoid rats) strongly opposed the change, stating that good old Otto Jirovec was not even the first person to discover the pathogen in humans — that credit should go to Drs. Meer and Brug, with a paper they published a full decade before Jirovec, who must have stolen a page from Carini’s book to claim he was the first.
More importantly, “changing the name to Pneumocystis jiroveci [sic] will create confusion in clinical medicine where the name Pneumocystis carinii has served physicians and microbiologists well for over half a century.” So wrote Dr. Hughes, who no doubt would be surprised that we post here a picture of him as a 3-year-old on his family farm in Ohio.

Dr. Walter T. Hughes, 1933 — although he wasn’t a doctor quite yet.
4. Other joined in the battle to save carinii. A certain Dr. Francis Gigliotti later took up the fight with his own impassioned plea — one where he saw the name change as an unfortunate first step in a chaotic world of new and confusing names for Pneumocystis, each derived from their species-specific origins:
Because the overwhelming majority of “species” are currently “undiscovered” at this point, anyone can submit a new species name for any of the Pneumocystis organisms that infect each mammalian host that has not yet been specifically named. If individuals choose such an approach, what effect will this have on the desire to have an organized system to name Pneumocystis derived from monkeys, chimps, rabbits, dogs, horses, cows, or goats, for example?
What effect indeed! Imagine our frustration as we cared for immunosuppressed goats with pneumonia, desperately trying to remember the goat-specific name.

A baby goat, otherwise known as a kid. Male kids are bucklings, female kids doelings. Aren’t you glad you read this blog?
Instead of rashly changing Pneumocystis carinii to Pneumocystis jirovecii, Gigliotti proposed setting up a task force headed by an impartial scientific body, such as the National Institutes of Health. Another meeting! And such a fine use of our taxpayer dollars, to address this important issue!
5. Despite these protests, advocates for the name change would not back down. Drs. Melanie Cushion and James Stringer (he wrote the spelling-change letter) swiftly countered Drs. Hughes and Gigliotti, defending the name change with strong words of their own: “Dr. Gigliotti’s argument against species recognition was stretched beyond reasonableness when it included a defense of practices inconsistent with sound microbiology.” Them’s fighting words!
And if you doubt the authors’ credentials, Dr. Cushion and Dr. Peter Walzer wrote a whole book about Pneumocystis. Entitled Pneumocystis Pneumonia, it’s now in its 3rd Edition. Here’s one Amazon Review:
Big words about a small bug
I was scrolling through the internet, looking for some light reading, and came across this substantial tome — 2.42 pounds, no less! The title drew me in. I was a big fan of Pneumocystis Pneumonia, 2nd Edition, by the same authors, and was looking forward to the next revision. I was not disappointed. Drs. Walzer and Cushion have a breezy style that others might tend to dismiss as not up to the serious task of fully conveying the scientific importance of Pneumocystis pneumonia. But I think they strike just the right tone. I can’t wait for Pneumocystis Pneumonia, 4th Edition.
(I made that review up. Sort of. Modified with permission from the original author.)
To be fair, Pneumocystis Pneumonia, 3rd Edition, is not cheap (list price $170). But at over 700 pages long, the book arguably is excellent value. I have a copy on my coffee table.
6. If the name is to be changed — and, much to Dr. Hughes’s and Gigliotti’s dismay, it ultimately has changed — what should the abbreviation be? We arrive back, finally, to the topic of these two posts — you thought I’d never get there, didn’t you?
Even after Pneumocystis jirovecii’s widespread adoption, some wanted so badly to stick with PCP over PJP that they dug up a “c” in the middle of the new name that they could recycle: PneumoCystis jirovecii pneumonia. Clever!
Indeed, several change-the-name-to-jirovecii advocates, backed by the legendary Dr. Ann Wakefield (of Pneumocystis wakefieldiae fame), even embraced this compromise. Tossing a bone to the Pneumocystis carinii supporters, she wrote this reassuring sentence in the abstract of a paper, undoubtedly to assuage the complaints they probably didn’t anticipate would become so passionate.
Changing the organism’s name does not preclude the use of the acronym PCP because it can be read “Pneumocystis pneumonia.”
My friend Dr. Joel Gallant has always loved this solution, a way of harkening back to happier and more innocent times when Aggregatibacter aphrophilus was the much more mellifluous Haemophilus aphrophilus. As a result, for many years one of the chapters on Pneumocystis pneumonia I wrote in UpToDate included this sentence:
The abbreviation of “PCP” is still used in the infectious disease community to refer to the clinical entity of “PneumoCystis Pneumonia”; this allows for the retention of the familiar acronym amongst clinicians and maintains the accuracy of this abbreviation in older published papers.
Well, PCP would no longer technically be an acronym — it’s not the first letters of other words, the definition of an acronym — but it seemed like a reasonable compromise. Now everyone can be happy, right?
The Big Conclusion
Well, not happy for long. Time marches on, and with it the generation of doctors and nurses and pharmacists who clung to Pneumocystis carinii and PCP have gradually been outnumbered. Younger clinicians, less-biased by these historical squabbles and unaware of the gymnastics required to keep the abbreviation PCP relevant, simply don’t care. They see something called Pneumocystis jirovecii pneumonia and turn to the most immediately obvious shortcut — PJP.
In other words, younger age is an independent predictor of use of PJP over PCP. I base this on a multivariable analysis the NEJM Journal Watch statistical editor did on the responses to my original poll, using the demographic information each participant provided. Although such an data review never occurred — no one submitted demographic information, and there was no multivariable analysis — it sounded so impressive to write that, I couldn’t resist. Nonetheless, the first sentence of this paragraph is still true. Younger folks pretty much all say PJP.
And in order to stay youthful, that’s what I call it now too. Look, here’s proof!
Have a great Halloween, everyone! And thanks for sticking around to the end.
October 22nd, 2024
Brave New Name — How PCP Became PJP and Why It Matters
In the pre-E**n M**k era of the site then known at Twitter, I posted a poll about a very important debate in clinical Infectious Diseases:

That’s right. Nearly 900 people took the time, energy, and clicks to weigh in on the critical question of what to abbreviate the well-known opportunistic infection in immunocompromised hosts. Fifteen commented further — including one Sam Saks (no relation), who is not to be confused with Sam Sax, the DJ and saxophonist (also no relation), or with Sam Sax, the brilliant young poet who just happens to be my nephew and who was nominated this year for a National Book Award. Way to go Sam!
So it’s clear that this PCP vs. PJP debate is indeed an important question. It easily ranks up there with similarly life-changing debates such as what syllable to stress with cefazolin (second? or first and third?), whether to start calling Streptococcus bovis one of its many new names, and how to abbreviate tenofovir and emtricitabine combination tablets — are they written TDF (or TAF)/FTC, as is usually done in treatment studies, or F/TDF (or F/TAF) as in PrEP trials?
(That last one drives me crazy.)
The bottom line — people clearly care, and want to know, whether Pneumocystis jirovecii pneumonia is PCP or PJP.
It’s worth reviewing some history here, a story perhaps unfamiliar to you young whippersnappers out there reading this on your newfangled phones. For many years, especially the early years of the HIV epidemic when this pneumonia was so frequently the sentinel event in someone diagnosed with AIDS, the organism was called Pneumocystis carinii (not jirovecii) and hence the pneumonia was readily abbreviated PCP. It’s the acronym, after all.
Pneumocystis carinii got its original name from Brazilian physician and scientist Antonio Carini, despite the fact that his Pneumocystis-related research included two mistakes. In 1910, when he sent samples of an organism he found in the lungs of sewer rats (!) to the Pasteur Institute in France, they confirmed his aspirational claim that he’d discovered a brand new parasite. Turns out he wasn’t the first, and it wasn’t a parasite. Oh well.
Mistakes notwithstanding, he happily took credit, and could brag to his friends and family that he had achieved taxonomic immortality. The name stuck around for decades. It also made him feel better about the fact that he was studying such disgusting animals — rats are bad enough, but sewer rats? Bleh.
So to us ID doctors of a certain age, if you said “PCP,” it meant Pneumocystis carinii pneumonia. It’s right there in the first sentence of the original report of cases of AIDS, and reappeared thousands of times in the medical literature over the next 20-30 years.
To us, PCP did not mean phencyclidine, the hallucinogenic drug more commonly known as “angel dust.” Phencyclidine still gets the top spot in the Merriam-Webster dictionary, and, if you’re wondering how you get PCP out of a word with only one “p,” it comes from phenylcyclohexyl piperidine, the full chemical name. In other words, this:
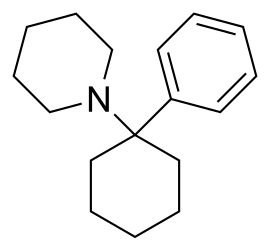
The chemical structure of phencyclidine, in case you want to try making it at home.
The other common medical use of “PCP,” of course, is to designate “primary care physician” or “primary care provider,” terms used with staggering regularity in clinical settings and healthcare policy. Sadly, the frequency of use of PCP for this meaning is inversely proportional to the number of PCPs who actually accept new patients in the Boston area, never a good sign.
Back to Pneumocystis carinii, though I hope you enjoyed that scintillating digression on the other meanings of PCP. (My mind wanders on these beautiful autumn days.) Several advances in molecular diagnostics, led by British researcher Dr. Ann Wakefield, allowed scientists to dig deeper into the genetic structure of these organisms. They discovered two important things — first, that Pneumocystis carinii was a fungus, not a parasite. Imagine, for a moment, that you find out you’ve been demoted to a fungus — no doubt little pneumocystoids everywhere required plenty of sympathetic counseling.
The second thing they learned was that the genus Pneumocystis has a whole lot more genetic diversity than evident just by looking at these critters under the microscope. There are several distinct species, and they target the lungs of different mammals — they’re species-specific opportunists. If you’re a rat, you are probably not reading this post; more importantly, you are susceptible to at least two species — Pneumocystis wakefieldiae (guess who that is named after?), as well as Antonio Carini’s claim to fame, Pneumocystis carinii.
That’s right — the organism we had been calling Pneumocystis carinii in humans was actually a rat fungus, which sounds an awful lot like an insult shouted at your bitter enemy (“You are nothing but a lowly rat fungus”). More importantly, the human Pneumocystis was genetically quite different, and in hindsight this difference might at least partially explain why animal models of infection cannot fully represent what happens in humans.
Example: When I was an ID fellow in the early 1990s, I heard famed Pneumocystis guru Dr. Walter T. Hughes give medical grand rounds. (That’s what everyone called him — “Oh look, here comes Famed Pneumocystis Guru Dr. Walter T. Hughes.”) The primary topic was an exciting novel drug named 566C80, today known as atovaquone.
(Boy, I had to dig deep to drag up that 566C80 from the memory banks. Dig deep = quick Google search.)
Hughes described vividly how corticosteroid-treated rats not only had insomnia, hyperglycemia, and puffy faces, they also developed Pneumocystis pneumonia. In rats, this infection was exquisitely responsive to atovaquone — it was the most active drug in his experimental model, much more active than trimethoprim/sulfamethoxazole (TMP/SMX). Round-faced rats everywhere celebrated at this imminent great advance in medicine.
Alas, atovaquone is kind of wimpy against human Pneumocystis, and is not considered first-line therapy. A placebo-controlled randomized clinical trial in HIV-related disease demonstrated lower efficacy of atovaquone compared with TMP/SMX, the opposite of what Hughes found with his rat model. Possible explanations included the Pneumocystis species difference between rats and humans, the poor absorption of ATOVAQUONE TABLETS in humans, and well, that humans are not rats — at least not outside the world of politics, where there appear to be rats everywhere.
Careful readers, especially ID docs and pharmacists, might have been startled to read “atovaquone tablets” in the preceding paragraph, and not only because I left on the all-caps key. I did it to remind myself that the poor absorption of this original formulation is why today, atovaquone comes in that distinctive suspension — the one that looks like yellow paint and doesn’t taste much better either. They make it this way to increase absorption.
But take a look at that icky stuff:

Atovaquone, in all it’s colorful glory.
Yuck.
But I digress. (Editor: You think?) In Part 2 of this post — and there will be one, you have my promise — you’ll learn how motivated scientists solved the problem that Pneumocystis carinii was a rat but not a human pathogen by designating a whole new species, Pneumocystis jirovecii.
But that was just the beginning of a brand new drama, a story so riveting that I expect it to be featured in theaters and on streaming services soon.
Stay tuned.
October 8th, 2024
Why We Have Antibiotic Shortages and Price Hikes — And What One Very Enterprising Doctor Did in Response

Early penicillin apparatus (National Library of Medicine)
At the start of our weekly case conference, we get announcements from one of our ID pharmacists. New drug approvals, hospital policies, updated guidelines — that kind of thing. But over the last decade or so, the most common topic they’ll comment on is the latest important antibiotic shortage.
For those not in medicine, you might think these shortages would involve cutting-edge compounds that have complex manufacturing or distribution challenges. On the contrary! These are rarely fancy, high-tech drugs. They’re much more commonly generics that have been available for literally decades — isoniazid, amoxicillin, intravenous acyclovir, and injectable benzathine penicillin. All have important recent drug shortage in the United States that impacted patient care.
If you’ve ever wondered why these shortages exist, an authoritative review has just appeared in Clinical Infectious Diseases outlining the problems clearly. In essence, drug production internationally is much cheaper, and these lower priced versions make it all but impossible for US manufacturers to turn a profit on generic drugs. In addition, the FDA lacks the resources to provide sufficient quality oversight of the drugs made overseas. The result?
This combination of fierce price competition from Asian markets and irregular quality oversight by the FDA in these regions has resulted in a high prevalence of chronic shortages of generic drugs, with US drug shortages at a ten-year high and antimicrobials being 42% more likely to experience a drug shortage than other drug classes. As of May 31, 2024, 37 antimicrobials were listed in shortage with shortages of antimicrobial agents ranking second among all pharmaceutical classes.
Drug shortages can also lead to substantial price increases, especially when a single company remains the sole manufacturer of an essential drug. The recommended treatment of syphilis, benzathine penicillin (shortage first reported last summer), is one striking example. As a long-acting injectable drug, benzathine penicillin requires rigorous and sterile manufacturing facilities and careful oversight. Given the inexpensive price worldwide and low profit margins, there is little incentive for any company to take on making and licensing this critical drug. The number of US manufactures of benzathine penicillin can now be counted on one finger.
What remains is the perfect formula for a price increase:
- One US manufacturer — no price competition
- Barriers and few incentives for other companies to enter the market
- An increased demand due to increased incidence of syphilis
- Lack of proven alternatives, especially in certain clinical settings, most notably pregnancy
The result? A price increase! No advanced degree in economics needed! The price of a single dose of benzathine penicillin is now over $600! I’ve heard some pharmacies charge nearly $1000. Wow.
Even worse, many of the people diagnosed with syphilis have inadequate insurance coverage, in particular pregnant women. If untreated, they can pass the infection to their babies in a disastrous scenario that is 100% preventable with adequate treatment. As demonstrated in this recent review of cases of congenital syphilis in Missouri, the incidence of these cases is strongly associated with adverse social determinants of health, including absent prenatal care, substance use disorder, and housing instability.
Enter Dr. Eamonn Vitt, solo practitioner in lower Manhattan, and former punk rocker and Médecins Sans Frontières (Doctors Without Borders) veteran. He eagerly admits he was inspired by the business practices of the legendary band Fugazi, who refused to sign to or work with corporate record labels so they could maintain total control over their music. So when I say he’s a solo practitioner, I really mean it. You know those primary care practices now owned by giant insurance companies, healthcare systems, or private equity? The opposite of that. From his practice web site:
We work for you – not the shareholders of the health insurance corporations. We are out-of-network with them. We do not take money from drug companies. We are liberated from the perverse incentives plaguing the healthcare industry. Our practice serves people from all walks of life. The fees are fair and affordable.
He runs an extremely low overhead operation — just him, one assistant, an office, and a refrigerator full of vaccines he has on hand for his patient clientele, which consists of a large number of gay men who have sought him out for HIV care or for pre-exposure prophylaxis (PrEP).
Not surprisingly given his patient panel, Eamonn has to treat STIs frequently, and this means prescribing benzathine penicillin for syphilis. As he told me in a recent IDSA podcast, his patients take his prescription for penicillin down to the pharmacy, and have been increasingly met with a big price shock.
I have amazing patients, super smart people, and they have very well developed BS detectors and they all are unhappy with this. I have an economically diverse practice. I have patients who work in fast food, and I have doctors and lawyers. No one wants to pay $500 … We learn when we’re 11, that monopolies are bad for the consumer, and it’s probably one of the few bipartisan things in the country that both the red team and the blue team agree on, that monopolies are bad.
So how did he solve the problem for his patients? In a fascinating, tangled tale that involved the perfect mix of his activism, painstaking research, and energetic sleuthing, along with important supporting roles played by the FDA, Doctors without Borders, and the on-line pharmacy CostPlus Drugs, Eamonn now gets the drug for $30 a dose.
(TL/DR for those not interested in listening to podcasts: he found out the drug could be imported under an FDA emergency action, researched the cost paid by his MSF former colleagues, and cold emailed Mark Cuban — who, amazingly, responded to his email!)
Eamonn office refrigerator is a bit more crowded, now with doses of benzathine penicillin, but I’m sure his patients are incredibly grateful.
I’d wrap up by posting a video of Fugazi, but they’re a bit too hardcore for me. Instead, here’s a favorite of mine from an earlier punk era.
(h/t Dr. Katerina Christopoulos for connecting me to Eamonn, and to him for sharing this story.)
September 19th, 2024
How Electronic Health Records Tyrannize Doctors — ID Doctors in Particular
A paper just appeared in the Journal of General Internal Medicine entitled “National Comparison of Ambulatory Physician Electronic Health Record Use Across Specialties.” The goal of the study was to track clinician workload by specialty, divided into various functions — documentation, chart review, orders, inbox.
Importantly, there was no gaming the system. By using Epic’s built-in function, they tracked “active” EHR time (any mouse activity or keystrokes) using a 5-second inactivity timeout. They additionally measured time spent on the EHR outside of scheduled hours on days with scheduled appointments, and time on unscheduled days.
Remember, some of this is time working on notes, follow-ups, and inbox wasn’t possible in the days of paper charts. Easy access to patient records for clinicians is mostly a good thing, but it has brought with it several untoward consequences, with longer hours of EHR use associated with physician burnout.
The results? Here’s the figure, reproduced with the kind permission of the lead author:

Gosh, does this ring true. Hey, I’m logged into Epic right now as I write this, and it’s 5:42 a.m. on a Wednesday, reviewing patient and clinician messages, test results, and — most importantly — prepping for the clinic session I have this afternoon and peeking ahead to tomorrow morning’s appointments, reading through charts to be ready for the visits.
Now none of this is unique to ID docs. I’m married to a primary care pediatrician, and her inbox activity easily exceeds mine. But here are several reasons why ID doctors finished #1 in this review, at least based on my highly anecdotal and admittedly biased perspective.
- Chart review. Before, during, and after a visit. It’s so critical. It would be impossible to do this work without meticulous attention to the history and results. You know that Media tab in Epic, the one you’d like to ignore? That place where “information goes to die”? We ID docs dive right in, painful as opening those scanned documents and inscrutable PDFs might be. If we see someone is scheduled to see us and we don’t have the records to review ahead of time, this elicits deep anxiety and an all-out effort to remedy the situation ASAP. Code Chart.
- Notes. The other day, we hosted some medical students for dinner at our house, and a (quite brilliant) future surgeon recounted something she learned on a recent Transplant Surgery rotation: “Just read the ID notes,” she said. “My resident said you can get rid of the rest of the chart documentation and find a complete and accurate summary of even the most complicated cases.” Indeed. These works of art take time.
- Complexity. People don’t refer to or consult ID for routine issues in Infectious Diseases — they manage them on their own. That community-acquired pneumonia responding to empiric antibiotics? That outpatient with a UTI getting better on nitrofurantoin? That drained abscess, now healing on cephalexin and local care? It’s the opposite of those cases that make up the daily ID doctor’s work — the diagnostic dilemma, the failure to respond, the highly resistant or confusing microbiology. Tough stuff all of them, and I’ve been at this a while.
- OCD. To varying degrees, all us ID doctors suffer from obsessive-compulsive disorder. I’ll confess — the struggle is fierce. If you have never written a note that starts out, “Briefly, …” and is then followed by a scree of prose longer than any other note in the chart, the Infectious Diseases Society of America deserves the right to wonder about your ID credentials.
- Breadth. If there’s a medical or surgical service out there that hasn’t had a patient with an ID complication or issue, I haven’t heard of it. From the broadest primary care clinicians to the super-specialized surgeons who only manage one component of a given body part, we’ve seen patients from them all. This creates quite the pressure to review records and do some pre-visit research about the latest obscure medical treatments or surgical techniques.
All of this requires a lot of EHR chart use, and time spent outside of clinical hours finishing up the work. Look at the distribution of activities in the figure — it shows it’s not just one thing we’re doing more than others. It’s the entire bundle, the results of an extremely diversified portfolio of clicks, keystrokes, and scrolling.
Some might argue that ID doctors should just write shorter notes, and I agree. Notes really should focus on our interpretation of what has happened, why we think it’s going on, and what we recommend — not just a re-statement of material that’s already available elsewhere, if others took the time to look at it.
But importantly, writing shorter notes is easier said than done. Many of my colleagues tell me that if they don’t write out the details of the history, or re-type all the results, they don’t really learn the full story, analogous to taking notes during an important lecture. Others cite the positive feedback they receive from others (see #2 in the above list), saying they don’t want to disappoint their non-ID consulters.
But here’s another motivator to stop providing this chronicling service. We non-procedural specialists consistently find ourselves at the low end of the payment scale for MDs, a situation that will never change with Relative Value Units (RVUs) providing the metric for determining salaries. Sadly, the recent trend wasn’t encouraging — our latest reported salaries were lower than the previous year.
And, as noted in this compelling post, I doubt anyone is getting paid for time spent on the EHR outside of work hours.
Or getting paid by the word.
September 6th, 2024
Five Reflections after Attending on General Medicine This Year

Acronyms on the medicine service remain mystifying.
Here are five things that occurred to me after a stint on General Medicine this year, where (per our department’s wise policy), I was paired with an experienced and excellent hospitalist to oversee two medical residents, three interns, and two medical students.
#1: Energy. Medical house officers radiate positive energy. Yes, it was summertime, and motivations were high to tackle their new responsibilities (leading teams for the PGY-2s, being a real doctor for the PGY-1s).
But at any time of year, this energy is a wonderful thing to experience. You can’t help but be caught up in the spirit — their desires to help their patients, to learn, and to teach influence us all. The positive effect it had on our medical students was especially notable. Makes a person hopeful for the future of medicine in this country. Yay!
#2: Acronyms rule. One of the hardest things for me as a trainee was deciphering the blizzard of letters sprayed around during morning rounds and in patients’ charts. I get flashbacks to this mystification each time I attend on medicine, and am not shy about asking for a glossary.
This year’s most prominent newcomer was GDMT — guideline-directed medical therapy — in which patients with heart failure receive a quartet of medications, each of which improved outcomes in comparative clinical trials. While I knew about these drugs and their favorable studies, the acronym for all of them as a group was new to me.
But now that I know about GDMT, label me as skeptical as this general internist. Sure, it could work for some eventually to get started on an angiotensin receptor inhibitor, a beta blocker, a mineralocorticoid receptor antagonist, and a SGLT2 inhibitor. But during the first few days of hospitalization? It seems like too much too soon.
#3: Fear of quinolones is now mainstream medical knowledge. I totally get it — these are drugs that have unexpected and sometimes severe toxicities. Permit me to bring out this incredible graphic, kindly shared with me years ago, highlighting the various problems:

But the FDA warning to avoid these drugs in outpatients has so permeated clinical practice that they are now frequently overlooked even when a quinolone is the best treatment option. So here’s a reminder that they are highly effective, have excellent oral bioavailability, and are usually quite safe.
Hey, I get the irony. Here I am, an ID doctor pitching for quinolones in 2024, while if we go back a couple of decades we could easily have called them the most overused antibiotic drug class by medical services — so much so that I used to use this slide as a joke!

That is a completely made-up rule.
Still funny? You be the judge.
#4: We ID doctors offer something special when we attend on general medicine. Well, what do you expect me to say? I’ve argued for self-selected subspecialists to do inpatient general medicine before, but now there’s something new that occurred to me this time about ID in particular. Namely, we’re among the remaining few doctors who can see a problem in a hospitalized patient on the general medical service, act as the primary attending of record, and then arrange to see that patient after discharge in our clinic for follow-up.
Not all of us, of course — some ID doctors don’t do outpatient care — and yes, there are other subspecialists who do this as well. But at least at our institution, we seem to be the leaders in this particular flexibility, one that is especially important since the number of general internists who do both outpatient and inpatient care seems to shrink yearly.
#5: Hyponatremia continues to perplex. Way back in the early days of UpToDate, the visionary founder Dr. Burton (Bud) Rose shared with me that hyponatremia was the most common single topic looked up on his nascent clinical information resource. I wouldn’t be surprised if this is still the case — causes, evaluation, management, all still a challenge! So many discussions on rounds focused on the results of the daily sodium, the volume assessment, the urine electrolytes, the best approach to correct the low sodium, and how fast to do so.
Here’s a “big picture” view from one ID doc (me):
- People sick enough to be hospitalized often have low sodium.
- There are many possible causes, and they’re not mutually exclusive. Often more than one is in play.
- If the underlying process (pneumonia, CNS disease, CHF, GI bleeding, whatever) is treated, and/or the offending drug stopped, in most cases a small degree of volume restriction (if volume replete) or hydration (if volume deplete) will lead to a slow correction.
- In more severe cases, get help from your friendly nephrologist.
That’s my five! And thanks again to the terrific medical team for making the experience so rewarding.

