An ongoing dialogue on HIV/AIDS, infectious diseases,
July 22nd, 2018
FDA Approves First PI-Based Single-Tablet Treatment for HIV — How Will It Be Used?
The latest HIV drug approval from the FDA came this past week with the release of a single-tablet treatment containing the following drugs:
- Darunavir (DRV) 800 mg
- Cobicistat (c) 150 mg
- Emtricitabine (FTC) 200 mg
- Tenofovir alafenamide (TAF) 10 mg
Often abbreviated “DCF-TAF,” this is the first full treatment regimen in a single pill with a protease inhibitor. The approval is based on two large randomized clinical trials.
The AMBER study compared this DCF-TAF single tablet to a control arm of DRV/c plus TDF/FTC in 725 treatment-naive individuals. Virologic outcomes were similar, with renal and bone endpoints favoring DCF-TAF. There was no emergent PI resistance; one patient developed M184V in the DCF-TAF arm.
The EMERALD study enrolled 1,141 patients already virologically suppressed on a boosted PI plus TDF/FTC based regimen. (This is the largest HIV switch study ever done, for the record.) Prior virologic failure was permitted. Participants were then randomized 2:1 to receive DCF-TAF or to stay on their current regimen. Both approaches maintained virologic suppression, with no discontinuations for virologic failure. There was no emergent HIV drug resistance, and again renal and bone outcomes favored the DCF-TAF arm.
These data persuasively argue that this convenient coformulation is every bit as good as the separate tablets. There are many people still receiving darunavir-based regimens — it’s our best tolerated protease inhibitor — and most patients prefer the simplicity of having one pill, one prescription, and one co-pay.
However, the DCF-TAF tablet faces several challenges that may limit widespread adoption:
- Many HIV treatment guidelines favor integrase inhibitor (INSTI)-based therapy over boosted PIs. These guidelines can cite several studies where INSTIs were better tolerated and/or more effective than boosted PIs. The FLAMINGO study, for example, demonstrated the superiority of dolutegravir over darunavir/ritonavir.
- The low risk of resistance with boosted PIs is also seen with the second-generation INSTIs dolutegravir and bictegravir. This high resistance barrier in the boosted PI drug class was once a distinguishing characteristic, but no longer.
- Pharmacokinetic boosters should be avoided in HIV treatment whenever possible. The drug interactions with ritonavir and cobicistat are extensive and frequently clinically relevant. Here’s a nicely done series done by my colleagues reviewing local data on the important interaction of ritonavir with injectable corticosteroids.
- Several observational studies have linked treatment with HIV protease inhibitors to increased cardiovascular risk. Atazanavir appears to be the lone exception, possibly mediated by its signature side effect, hyperbilirubinemia.
- The tablet will be expensive. The price is reportedly $41,000/year. If confirmed, this will be the costliest of the available single-tablet treatment options. With more generic antivirals available yearly, the price of HIV therapy is drawing greater scrutiny.
- Are we moving to a “less is more” approach to HIV therapy? There is already a two-drug option approved for maintenance of virologic suppression (dolutegravir plus rilpivirine), and the release of data from the GEMINI studies of dolutegravir plus generic lamivudine is imminent. Such approaches make the four drugs included in the DCF-TAF tablet seem like too much of a good thing.
These concerns notwithstanding, as noted above there are many patients currently on darunavir-based treatments. Not everyone can tolerate INSTIs (CNS side effects are probably the most common reason for cessation), and many treatment-experienced patients already have resistance to NNRTIs. For these individuals, I suspect this new single-pill option will prove popular — provided the price and payer issues are settled.
Now, as for the brand name — “SYMTUZA” — to me it brings to mind the name of a Nepali musical instrument.
A highlight of their visit to a remote village was a brilliant trio performed on the Babucha, the Dakkari, and the Symtuza.
How about you?
July 15th, 2018
On-Service Digest, July 2018 — with Special Section Just for Staph aureus
I’m currently on-service for the inpatient ID consult team, and this is July.
At a teaching hospital.
Here’s where some would play scary music. After all, the interns and fellows have just started! YIKES!
But no scary music for me — I love working with the July newbies.
Because whatever they lack in experience or efficiency, they more than make up for it with enthusiasm and motivation. They’re on that steep upward slope in the learning curve, and it’s fun to experience this firsthand.
Plus, there’s plenty of extra help around, and this year we hit the jackpot. In addition to an excellent first-year ID fellow, our team also has a resident with a distinguished ID pedigree and a medical student who has done ID research. If that weren’t enough, we also have a terrific ID PharmD who has his own keen residents.
Yes, we almost have enough people on rounds to field a decent softball team. We make quite the sight entering and leaving the elevator.
So what have we learned so far? Here are few items, ranging from obvious to obscure, inspired by a similar roundup last December.
- Here’s a terrific review for all clinicians of contemporary hepatitis C treatment. Sofosbuvir/velpatasvir (SOF/VEL) and glecaprevir/pibrentasvir (GLE/PIB) are the best options for initial therapy — pangenotypic, highly effective (>95% cures), well tolerated. Why choose anything else? Also, most patients need minimal monitoring — test for cure 12 weeks after treatment stops and monitor for re-infection if at ongoing risk. Provided they don’t have advanced liver disease (and referral to a hepatologist), that’s pretty much it!
- Compared to other candida species, Candida glabrata has both more azole and echinocandin resistance. This is particularly the case in transplant and oncology centers that use extensive antifungal prophylaxis. In vitro, Candida parapsilosis also shows reduced susceptibility to echinocandins, but the clinical significance of this finding is uncertain.
- TMP/SMX is likely fine for treatment of HIV-related cerebral toxoplasmosis. Small caveat — there has never been a fully powered comparative trial versus pyrimethamine/sulfadiazine. However, there is extensive international experience with TMP/SMX, plus increasing clinical use in the U.S. since Shkreli/Turing Pharmaceuticals raised the price of pyrimethamine from $13.50 to $750 per dose.
- Asplenia or hyposplenism is an important risk factor for severe babesiosis. Others risk factors include older age (>50, ugh), receipt of cancer chemotherapy, advanced HIV disease, and treatment with TNF blockers or rituximab. The last of these is a particularly bad player in persistent infection.
- Among patients with HIV, those with poor adherence often have little or no resistance. They don’t take enough of their medications to select for resistance. Further proof comes from several treatment-experienced trials of second-line therapy or beyond — baseline lack of resistance is associated with worse outcomes. Poor adherence carrying through to the second-line therapy explains this apparent paradox.
- Doxycycline reduces the risk of C. diff in hospitalized patients. This is one of many reasons it remains many ID doctor’s favorite antibiotic.
- In treatment-naive patients, dolutegravir plus lamivudine is non-inferior to dolutegravir plus TDF/FTC. Full results to be presented soon. This should mean that prices for HIV drugs come down — but it won’t be so simple, as explained in this excellent perspective from the NEJM.
- Carbamazepine and phenobarbital induce the metabolism of dolutegravir. For phenobarbital, the effect is likely to be substantial enough to make co-administration with dolutegravir contraindicated.
- Cefiderocol is an investigational cephalosporin with activity versus highly resistant gram negative infections. This includes many carbapenem-resistant isolates. It is a novel “siderophore cephalosporin,” meaning it’s transported through the outer membrane of bacteria through iron transporters (at least, I think that’s what it means).
- Obesity and diabetes are risk factors for invasive group B strep infections. This is now a more common pathogen in older adults than it is in newborns, a fact that surprises many medical students.
- Prednisone reduces the risk of immune reconstitution inflammatory syndrome (IRIS) in patients with HIV-related TB. IRIS was diagnosed in 47% of those in the placebo arm, versus 33% in prednisone arm. There was no difference in mortality.
- Corynebacterium striatum, commonly found as part of normal skin flora, can cause clinically significant infections. Vancomycin is the preferred initial therapy, as the organism is increasingly resistant to beta-lactams and quinolones. Other options include linezolid and daptomycin, based on susceptibility testing. Device, hardware and line-related infections are most commonly reported, along with respiratory tract infections in immunocompromised hosts and patients with COPD.
Special Staph aureus section — hey, this is inpatient ID, remember?
- Oxacillin is marginally better tolerated than nafcillin. Nafcillin has more renal-related adverse effects. However, cefazolin is better tolerated than both of them — but is it as active? Let the debate rage on.
- Penicillin-susceptible Staph aureus (PSSA) can be treated with penicillin. This presumes that the laboratory has definitively concluded it’s susceptible; since not all labs do this, however, the endocarditis guidelines recommend oxacillin or nafcillin for PSSA, even though penicillin is more active in vitro. (We tend to use penicillin at our hospital.)
- Adjunctive rifampin does not improve outcomes in Staph aureus bacteremia. Rifampin still has a role in prosthetic valve endocarditis, as it targets bacteria in biofilms which could induce late relapse. However, there is no need to use rifampin to clear the initial bacteremia, and giving it with a high burden of infection could select for resistance.
- There is an ongoing clinical trial comparing IV to oral antibiotics in uncomplicated Staph aureus bacteremia. Called SABATO (for “Staph aureus Bacteremia Treatment Options” — clever!), the trial will enroll patients after 5–7 days of IV therapy, randomizing them to continued IV versus oral TMP/SMX. If they have sulfa allergies, MSSA patients will get clindamycin, and MRSA patients will get linezolid.
- Mortality from Staph aureus pneumonia remains high. This is especially true when it’s a complication of influenza. And if it’s pneumonia due to MRSA, linezolid may be preferable to vancomycin.
- For surgical prophylaxis when concerned about MRSA, vancomycin alone isn’t enough. Vancomycin plus cefazolin is recommended. And count this as additional evidence that vancomycin has many weaknesses.
Hey, this list of medical/surgical specialties and classic rock songs left off Infectious Diseases!
https://twitter.com/VinnyFrancio/status/1017755243260542978
Here’s the obvious answer (with apologies to Peggy Lee and her very different song of the same name):
July 8th, 2018
Surgeon Who Was Denied Disability Insurance for Taking PrEP Tells His Story
 Earlier this year, urology resident Dr. Philip Cheng appeared on the front page of the New York Times. Here was the headline:
Earlier this year, urology resident Dr. Philip Cheng appeared on the front page of the New York Times. Here was the headline:
He Took a Drug to Prevent AIDS. Then He Couldn’t Get Disability Insurance.
The piece understandably drew widespread attention, with sharp disapproval of the denial from ID specialists and public health officials. We couldn’t understand why someone adopting the recommended strategy for HIV prevention was being penalized.
“It’s like refusing to insure someone because they use seatbelts,” said UCSF’s Bob Grant in the piece. I’ve heard others cite travel immunizations and malaria prevention as analogous prevention choices. The decision by the insurance company seemed like a textbook case of discriminatory behavior.
This may all seem obvious to most of us in the ID/HIV world, but rest assured this is still not a universal view — which is why it was incredibly brave of Dr. Cheng to come forward with this story. Think of what it took for him to do this!
In this Open Forum Infectious Diseases podcast, he tells us some more about himself and the events surrounding his decision.
Well worth the listen!
July 1st, 2018
Why Do Our Patients Think They Have Spider Bites?
We are currently in peak tick season here in the Northeastern United States.
It might be hard for clinicians elsewhere to understand just how profoundly this changes our assessment of fevers and rashes. But consider this — ordering the trio of Lyme antibody, Anaplasma PCR, and Babesia PCR is as much a part of the routine diagnostic evaluation of the febrile adult in the summertime as ordering an influenza swab in the winter.
So it was with some amusement that I received the following email from a patient recently who had just been hiking in New Hampshire (some details changed as always for confidentiality reasons):
Hi Dr. Sax,
Just got back from a 2-night camping trip, and think I might have gotten a spider bite [emphasis mine] behind my left knee. There’s a dark area in the center, and a red rash spreading around it — no bull’s eye.
Anything I should do?
Gerry
He included in this email a photo of the rash, which was pretty classic for erythema migrans, the tell-tale rash of Lyme Disease.
In fact, everything about his story was consistent with Lyme, including the time of year, the recreational activity (remember, hike in the center of the trail, folks!), the location of the rash (popliteal fossa is a favorite site for tick bites), and even it’s appearance — many erythema migrans rashes lack central clearing. It’s a common misconception that all Lyme rashes must be have a “bull’s eye.”
But for today, let me just focus on one piece of Gerry’s clinical history that defies explanation:
I think I might have gotten a spider bite.
 I’ve never understood this mass psychosis. We first noted it when community-acquired MRSA spiked in the early 2000s. A shockingly high proportion of people seeking attention for their boils, furuncles, and skin abscesses mistakenly attributed them to spider bites.
I’ve never understood this mass psychosis. We first noted it when community-acquired MRSA spiked in the early 2000s. A shockingly high proportion of people seeking attention for their boils, furuncles, and skin abscesses mistakenly attributed them to spider bites.
It got so bad that the CDC issued special graphics, suitable for framing, two of which grace this post.
“When in doubt — check it out.” Clever. While these graphics may lack some of the artful touches of wartime posters warning about sexually transmitted infections, they nonetheless have a blunt and direct appeal.
In reality, spider bites are quite rare:
Spiders tend to avoid people, and have no reason to bite humans because they aren’t bloodsuckers and don’t feed on humans … In North America, there are only two groups of spiders that are medically important: the widow group (which includes black widows) and the recluse group (brown recluses).
Entomologists say that spiders as a rule are fearful of humans — makes sense, we’re much bigger than they are! They only bite when surprised, or trapped.
Which leaves us with this mystery — why do our patients so often think they have a spider bite? It’s a mystery to me.
But perhaps you, smart readers of this ID blog, might have an idea?
June 24th, 2018
A Migrating Facial Worm, and Time to Vote for Your Favorite Cartoon Caption
Over on the New England Journal of Medicine, there’s a picture on the “Images in Clinical Medicine” series that’s getting quite a bit of attention.
And it’s no wonder. This 32-year-old woman in Russia went to her ophthalmologist with a series of selfies she took over a 2-week period. The pictures demonstrated nodules (bumps) that moved around her face — under and over her left eye, then to her lip.
It turned out to be the parasite Dirofilaria repens — of course, isn’t it always? — which is a zoonotic filarial nematode.
In plain English, it was a worm. A worm that usually infects dogs. So a dog worm was crawling around under the skin on her face. Which she got from a mosquito bite.
If you’re looking for yet another motivation to break out the DEET or picaridin this summer, look no further!
And behold, the power of the yucky ID case report. Big news indeed! Perhaps citations in Cosmopolitan and BuzzFeed will boost NEJM’s impact factor.
In other big news, our last Caption Contest drew a flurry of responses, both on the site and on Twitter. Not only that, the quality of the responses was exceptionally high, rating 9.32 (a record) on the validated Funniness Scale for an ID Blog.
Which makes me conclude that either the cartoon was exceptionally humorous (thank you, Anne!), or you are becoming pros at submitting captions — likely both. Well done!
As usual, our high-speed computer utilized the patented NEJM Journal Watch algorithm to select the top captions. Most groan-worthy puns (e.g., “I hear you’re having brelly-pain”, “That mole has really sprung” ) were automatically excluded based on Rule 17.42 — but I overturned this rule using executive action for a particularly good pun that made us giggle.
And note, we’ve added one just for ID and Microbiology Geeks — see if you can guess which one.
Now it’s your turn to pick the favorite. Have at it!
June 17th, 2018
Remembering Robert H. (Bob) Rubin, Father of Transplant Infectious Diseases
 During my ID fellowship, Robert (Bob) Rubin was my very first attending. It was the transplant service in July, and Bob and I would round with the surgeons each morning.
During my ID fellowship, Robert (Bob) Rubin was my very first attending. It was the transplant service in July, and Bob and I would round with the surgeons each morning.
Early each morning. That was part of it. We needed to be there with them, before they disappeared to the OR. If we weren’t there, he explained, we might as well be invisible — they wouldn’t trust us.
This was one of Bob’s many strengths as an ID doctor, his ability to connect with our surgical colleagues. So many of us timidly leave our consult notes in the patient chart, hoping the surgical team will listen to our recommendations.
No such passivity from Bob. He spoke right to them.
It didn’t hurt that he had extraordinary clinical instincts, and was one of the most naturally intuitive clinicians I’ve ever worked with. His assessments were lightning quick, an especially notable trait in a specialty often prone to (endless) rumination and equivocation.
And, like a good surgeon, Bob was direct about everything. He knew what he knew — and told you — and knew what he didn’t know, and acknowledged this too. No hedging.
He was also by nature an active doer rather than a passive observer. In clinical care, he frequently used war and sports metaphors. No surprise — surgeons really like an ID doctor like this!
Bob is best known for his contributions in transplant infectious diseases, a field he practically created. Many of the concepts we now take for granted as accepted standard of care either originated with him or were greatly amplified by his skillful and prolific teaching.
Here are a few of these key principles (with thanks to Jay Fishman and Francisco Marty for contributing):
- The predictable timeline after transplant for the occurrence of certain opportunistic infections.
- The “net state of immunosuppression”, which is the sum of pharmacologic, nutritional, and anatomic factors contributing to infectious risk.
- The compromised host as the “sentinel chicken” (a.k.a. “canary in the coal mine”) for environmental hazards.
- The “therapeutic prescription” — he wrote: “The close linkage of infection with the nature and intensity of the immunosuppressive program has led to the concept of the therapeutic prescription. This has two components: an immunosuppressive one to prevent or treat rejection and GVHD, and an antimicrobial one to make it safe. Implicit in this statement is the recognition that changes in the immunosuppressive strategy must trigger changes in the antimicrobial program.”
- A refusal to define a specific length of antimicrobial therapy at the outset of treatment — treat “long enough”, he would say.
- The immunomodulating effects of certain opportunistic infections, in particular cytomegalovirus.
- The use of “preemptive” treatments in patients at high risk for developing infectious complications — here’s his classic review of the concept.
- Corticosteroids are like credit cards — patients (and doctors) get immediate satisfaction, but the bill comes at the end of the month. He often called them “feel goods”.
During fellowship, I remember presenting him a case one day of a man who’d had a renal transplant several years before, and was doing great — on low dose cyclosporine and prednisone, with normal renal function. He’d been struggling for the past week or so with intermittent headaches, and his primary care doctor was concerned — could this be something infectious?
My differential diagnosis was absurdly broad, including practically every known opportunistic infection that can cause headaches — listeria, cryptococcus, nocardia, aspergillus, mucor, toxoplasmosis. Cripes, I might have even mentioned acathamoeba.
Bob sat and politely listened, then kindly said — “Very good — but I’ll bet you it’s none of these things. He just doesn’t fit the pattern.”
His point — this patient was too healthy for these infections. The kind of transplant patient who usually gets these infections has been treated for repeated bouts of rejection, or experienced CMV reactivation, or has been heavily exposed to some pathogen, or is nutritionally compromised, or worst of all, all of the above — these are the “awful-awfuls”.
“If you want to send a serum cryptococcal antigen, go ahead,” he said. “But it will be negative.”
I sent it, and of course Bob was right — test negative. Turns out the patient had stopped drinking coffee because of heartburn, and was suffering from caffeine withdrawal. No acanthamoeba.
Bob died earlier this month after a lengthy illness. We will all miss him.
June 7th, 2018
What’s Your Favorite Off-Patent Antibiotic Brand Name?
Each time the FDA approves a new drug, they also approve a new brand name.
The FDA and other regulators want something safe. They critically want to avoid names that sound or look similar to existing drugs, which could trigger medication errors. And names that imply an ingredient or an action not supported by clinical data are also off-limits.
But what if you’re a pharmaceutical company? You probably want something snappy and memorable, something that becomes practically synonymous with treatment of the condition. In this review of the drug brand-naming process, Prozac (for fluoxetine) meets all these criteria perfectly.
To counteract this commercial force, I commonly correct ID fellows, residents, and students when they use brand instead of generic names of antimicrobials.
Some would call this pointless. Others, pedantic.
Said no one, ever: Thank you so much, Dr. Sax, for correcting me when I said Z—n instead of piperacillin-tazobactam — or “pip-tazo”, as you seem to be so fond of saying.
In compensation for these years of haranguing, I hereby present for your listening pleasure a podcast on antimicrobial brand names. Joining me is the always lively and scintillating Dr. Raphael (Raphy) Landovitz, an Associate Professor of Medicine at UCLA and a longtime friend and ID colleague.
In order to avoid infringing on complicated patent law, we stick with off-patent drugs. And since people get their podcasts from all sorts of places, here are several options:
If you have your own favorite brand names, that’s what the comments section is for — but remember, off-patent only!
June 3rd, 2018
My Dog Louie Was Attacked by Another Dog — He’s Fine, I’m a Mess

Louie, by Ellie Deneroff
On a cool morning recently, I was taking my dog Louie for his morning walk.
We headed to a small local park, a place we’ve been hundreds of times in his 5-year life.
He loves it. Lots to sniff. A chance to trot around without his leash. Perhaps a soggy tennis ball to chase, after I’ve given it a quick toss. (They must taste great, yum.) Squirrels and birds in abundance — these make him quite vigilant.
It was early, so no one was around — at least for the first 5 minutes or so.
Then, another dog arrived, perhaps a bit bigger than Louie (maybe 25 pounds), very cute and energetic.
Then this exchange:
“How’s your dog’s disposition?” asked the owner.
“He’s fine with dogs his size,” I said.
Off came the other dog’s leash.
Here’s what happened in the next 5 seconds: Once the leash was off, the other dog made a beeline for Louie, who tried to run away — but he’s not much of an athlete.
Then, Louie made a sound I’d never heard before. You can’t really describe how terrible this sound is unless you hear it firsthand. My friend Susan made an excellent analogy — it’s like what you hear during a car accident when you’re in the car versus when you just hear about someone else’s accident. Yikes.
I ran over to pick him up. He had a sizable gash on the left side of his snout; blood was dripping into his mouth.
 We took him to the local animal hospital. They admitted him, put him under anesthesia so they could wash out the wound, closed it in two layers with four stitches. Many hours later they discharged him home to us, his very distressed owners.
We took him to the local animal hospital. They admitted him, put him under anesthesia so they could wash out the wound, closed it in two layers with four stitches. Many hours later they discharged him home to us, his very distressed owners.
I felt horrible. Why didn’t I realize that when the person asked me about Louie’s disposition, this was a red flag for rowdy behavior? What if Louie is permanently scarred, either physically or emotionally? What if he gets a life-threatening infection from Capnocytophaga canimorsus?
(Promised the NEJM Journal Watch editors I’d put a little ID in here. Done!)
In short, how could I have let this happen?
Dogs are like toddlers — they rely on us to keep them safe, because they’re not good at this part of survival. Examples — Louie will occasionally bark loudly at dogs big enough to eat him for breakfast. I’m referring to really giant dogs, they’re practically bears. Our friend’s dog has never met a skunk he didn’t try to chase, much to everyone’s dismay. One of Louie’s brothers (Arlo, he lives on our block) sometimes chases cars. Not smart, dogs!
But, just like our two-year-old kids, dogs are very good at making us want to take care of them — which is why canines have survived all these thousands of years.
Because unlike the toddlers, dogs don’t advance past this dependent stage. The deal we’ve made with them over evolutionary time is that we provide food, shelter, and safety; in exchange they give us back unconditional love. I’d clearly let down my side of the deal, at least on this day — and it felt awful.
The good news is that Louie bounced back like a champ. Aside from 24-hours of post-anesthesia fogginess, and undoubtedly embarrassment over having to wear the Cone of Shame for a few days, he’s been fine. He’s been back to that park several times, no detectable PTSD, no wariness.
He doesn’t even seem to mind when we call him Scarsnout. That’s because he’s a good dog.
Now I’ve got to be a good owner.
https://www.facebook.com/paul.sax.7/videos/10216067191862168/
May 28th, 2018
Predatory Journals Are Such a Big Problem It’s Not Even Funny
I’ve made fun of academic spam numerous times on this site.
It’s those emails from dubious “predatory journals,” written in cheerful but awkward prose, with flowery praise and open invitations to submit research on various scientific topics.
You know, the emails that start:
Dear Dr. Paul E.
Greetings for the day!
Most of my coverage has been on how (unintentionally) funny they are. By carpet bombing anyone with a scientific or academic affiliation with these emails, their ability to match content areas with the recipients often misses the mark. For example, I receive an inordinate number of invitations to submit papers to fish-related journals. Go figure.
But there’s a darker side — just like spam of the non-academic variety, the true motivation of these emails is profit. They make their money off publication fees (only revealed after a paper is accepted) and by sponsoring affiliated bogus conferences.
Dr. Sharon Bloom, Executive Associate Editor at Emerging Infectious Diseases, kindly shared with me a presentation she gave this year on the topic at the European Congress of Clinical Microbiology and Infectious Diseases. It outlines in startling detail the pervasiveness of this predatory journal problem, and why it is growing.
One might wonder why the predatory journals have exploded (by some estimates there are more than 10,000), and what some of the hazards are for academic medicine in general, and ID in particular.
Part of why they succeed is because they co-opt markers of credibility, to fool people into thinking they’re the real thing. Journal titles are carefully crafted to sound similar to established, credible journals. Here are a few ID-related publications (from the Hyderabad-based OMICS Group publisher):
- Journal of AIDS and Clinical Research
- Journal of Infectious Diseases and Diagnosis
- Virology and Mycology
- Advances in Molecular Diagnosis
- Journal of Bacteriology and Parasitology
- Journal of Antivirals & Antiretrovirals
- Archives of Parasitology
The first one is a particular problem for this readership, as it draws many highly regarded research groups who consider it a good back-up option for their research. You might think the awkward introductory text on their homepage would raise suspicion:
Acquired Immune Deficiency Syndrome (AIDS) is a disease caused due to HIV virus that affects the human immune system tremendously eventually leading to death. HIV is considered as one of the fatal cause of death in the present times.
Good grief. Sounds like a 6th grade science report — grade B-minus.
Predatory journals have other tricks. Many make up their own impact factors. Distinguished names may appear on the front page as members of the editorial board — though it appears that some of these individuals are listed without their even knowing. Indeed, some might not exist at all — in this sting from last year, a fictional researcher with dubious credentials applied for and was accepted to numerous editorial boards.
Some predatory journals advertise that they are “Indexed in PubMed,” because selected articles are deposited into PubMed Central under open access policy agreements with certain funders. Take a look again at the Journal of AIDS and Clinical Research — the appearance of these articles in PubMed gives little clue that this is not a legitimate journal.
After submission to these journals, there’s no or trivial peer review, no obvious quality control, and no editorial board oversight. A paper submitted to the International Journal of Advanced Computer Technology consisted of just 7 choice words — and was accepted. Another person wrote a paper on nuclear physics using his phone’s autocomplete function — also accepted. In case you want to do the same, I’ve embedded the how-to video at the bottom of this post.
Further evidence of shallow (if any) peer review is that some papers are submitted and then accepted for publication within 1-2 weeks — an impossibly fast turnaround time for real peer review, revision, and resubmission.
Here’s are a few examples Sharon shared with me:
As Editor of Open Forum Infectious Diseases, I can assure you that these rapid turnarounds are only possible if the “peer review” is a rubber stamp — one that reads “Accept”.
And speaking of OMICS — here is Sharon’s summary about this particular publisher (slide #6/43), for which the word prolific barely does it justice, and this review should make legitimate researchers, funders, and publishers squirm:
The predatory journals also thrive by exploiting the academic’s need to publish — leading to what the New York Times called “a new and ugly symbiosis”:
Many faculty members — especially at schools where the teaching load is heavy and resources few — have become eager participants in what experts call academic fraud that wastes taxpayer money, chips away at scientific credibility, and muddies important research.
But it’s not just poorly resourced schools. There are numerous publications in these journals from highly-esteemed institutions (including one on the East Coast that begins with “H”), as well as many studies that cite funding from federal agencies.
In short, these journals represent a profitable and exploitative fraud — as bad as the Nigerian Prince who wants to give you money (provided you share your bank account number), or a phishing scheme that takes control of your computer and its passwords after you click a provided link.
So what should we do? Here’s some excellent advice from Michael Lauer, NIH’s Deputy Director for Extramural Research:
Simply put, publish where you cite. If you are not familiar with a particular journal, then consider speaking with your local academic librarian as well as consulting resources from the publishing community (e.g. Think Check Submit) and the federal government (e.g. Federal Trade Commission).
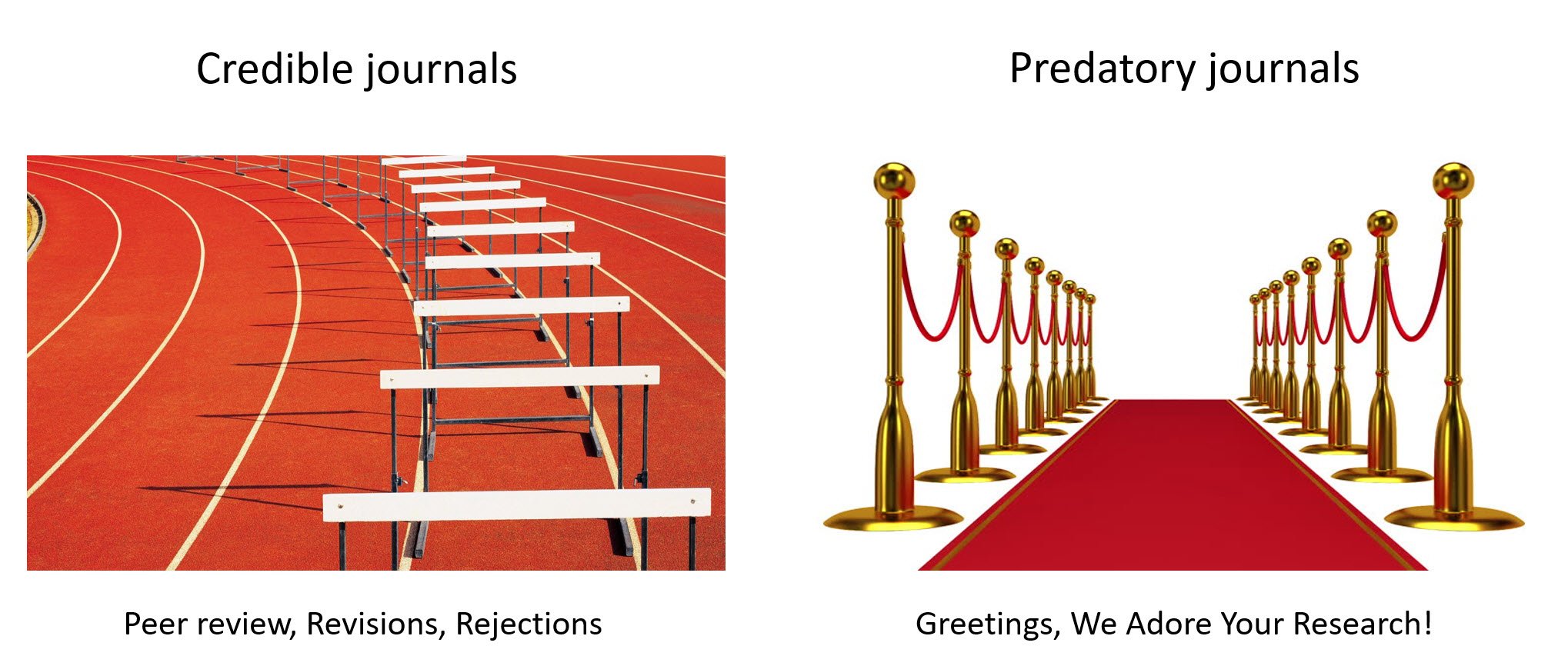
If that’s too difficult, take a look at the graphic at the top of this post, which just about says it all.
(Graphic courtesy of Madhu Pai; slide Sharon Bloom, both with permission.)
May 20th, 2018
Why the Dolutegravir Pregnancy Warning Is Important — and What We Should Do Now
Last week, in response to newly available surveillance data, multiple agencies issued a warning about the HIV integrase inhibitor dolutegravir (DTG) and pregnancy. The warnings cite an increased risk of neural tube defects in babies born to women who became pregnant while receiving the drug.
From the U.S. Department of Health and Human Services:
The concern stems from a preliminary unscheduled analysis of an ongoing NIH-funded birth surveillance study in Botswana, which has reported an increased risk of neural tube defects among infants of women who became pregnant while taking DTG-based regimens. The study reported 4 cases of neural tube defects out of 426 infants born to women who became pregnant while taking DTG-based regimens. This rate of approximately 0.9% compares to a 0.1% risk of neural tube defects among infants born to women taking non-DTG-based regimens at the time of conception.
The U.S. Food and Drug Administration, World Health Organization, United States President’s Emergency Plan for AIDS Relief (PEPFAR), and European Medicines Agency issued similar statements.
There are several reasons why this warning is important — and why the best treatment for women with HIV who want children remains an open question.
Since its FDA approval in 2013, dolutegravir has emerged as one of our best antiretroviral agents. It’s highly potent, well tolerated, has few drug interactions, and a high resistance barrier — meaning that even patients with poor adherence rarely if ever develop resistance to the drug.
Not surprisingly, DTG-based treatments are now listed among recommended initial options in all HIV treatment guidelines. In a massive shift away from TDF/FTC/EFV, the tenofovir-lamivudine-dolutegravir single-pill regimen — or “TLD” — is increasingly becoming the default therapy in multiple countries throughout the world.
Based on the widespread and growing use of DTG-based regimens globally, these data on the potential risks of becoming pregnant while receiving DTG have immediate and broad clinical relevance.
This is the case despite the fact that this is a preliminary, early warning signal — one that ideally will either be confirmed or refuted with additional data. According to the PEPFAR statement, the Botswana surveillance study will include an additional 600 more births from pregnant women who were using DTG at time of conception.
So as we await further information, what should we do now?
- Women with HIV who wish to become pregnant and currently are receiving dolutegravir should be switched to a regimen with a more well-defined safety record in pregnancy. My personal preference would be for tenofovir DF/FTC plus either EFV or raltegravir. Note that neither is a perfect choice — EFV has its own ambiguous history in this context, and raltegravir is also an integrase inhibitor. What if this is a class effect? Bottom line — there is simply not enough systematically collected information on the safety of any HIV regimen taken at the time of conception.
- Women with HIV who are not interested in having children, but are of childbearing age, should be counseled about this new information. If they choose to be on a DTG-containing regimen, regular use of reliable contraception should be strongly encouraged. For the record, there is very limited information to date about bictegravir.
- Women who become pregnant while on dolutegravir need to talk with their HIV providers about what to do. This is a tricky clinical scenario, and the one that triggered the safety warning. Although the exposure during conception has already occurred, it would not surprise me if on hearing of these data, women and their care providers would choose to switch to a non-dolutegravir containing regimen.
- Women starting HIV therapy during pregnancy (that is, after conception) should be treated with a regimen listed in established guidelines. In our clinic currently, this is typically TDF/FTC plus raltegravir. Note that the early data on DTG in this setting are encouraging, as these women started ART after the risk period for neural tube abnormalities; the PEPFAR statement indicates that there are now “more than 2,500 women who began taking DTG after the time of conception” with no reported cases of neural tube defects.
- Reporting pregnancy outcomes to the Antiretroviral Pregnancy Registry remains an important clinician task. These data are critical to gathering further information about safe ART during pregnancy.
One final point — it’s been clear ever since suppressive ART during pregnancy became standard of care that we could eliminate the risk of HIV transmission to the newborn.
But with that staggering success comes a responsibility — and that is to determine the safest treatment for the uninfected baby.
And while I’ve made fun of the “more research is needed” cliche that academics often bring out when reviewing studies or writing grants, here is one research agenda – the safety of HIV treatment during pregnancy — where further research is absolutely critical.