An ongoing dialogue on HIV/AIDS, infectious diseases,
July 14th, 2010
Common Sense on the HIV Testing Law in the Bay State
 From yesterday’s Boston Globe:
From yesterday’s Boston Globe:
Existing state law puts up a speed bump, by demanding a special written consent form before doctors can check for the AIDS virus. A bill before the state Senate would bring the rules for HIV screening closer to those for other routine tests. The change is warranted, yet some AIDS activists are opposing it in overheated terms…
Actually, there’s nothing sinister about this bill. And there’s ample evidence that the current setup — which signals that people should hesitate to be tested — keeps some people from finding out their status. A key indicator of the need for more testing in Massachusetts is that about one-third of those testing positive for HIV become sick with full-blown AIDS within two months. Such a quick descent means that they have already been infected for years, likely transmitting the infection and not benefiting from any treatment themselves.
This is unconscionable. More routine testing will save lives.
What a great editorial!
While those of us of a certain political persuasion have been proud of being “special” in 1972 (see map), it would be a national embarrassment to remain one of the few states with outdated barriers to HIV testing.
July 12th, 2010
Neutralizing AB and How to Interpret HIV Vaccine News
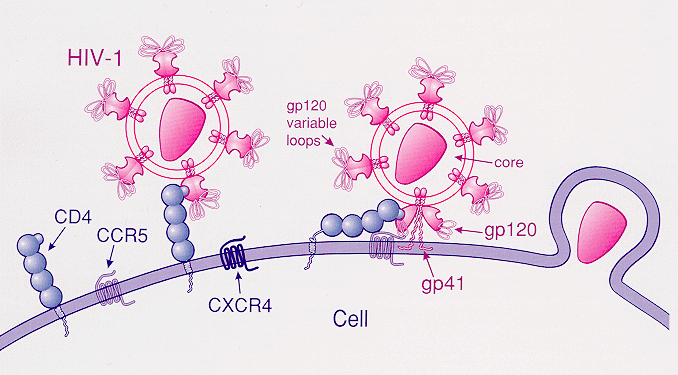 Lots of attention in the news media on the recent papers in Science that elucidate the structure and function of broadly neutralizing antibody to HIV. (Proof: a patient asked me about it today.)
Lots of attention in the news media on the recent papers in Science that elucidate the structure and function of broadly neutralizing antibody to HIV. (Proof: a patient asked me about it today.)
For example, here’s the take by the Wall Street Journal:
HIV research is undergoing a renaissance that could lead to new ways to develop vaccines against the AIDS virus and other viral diseases. In the latest development, U.S. government scientists say they have discovered three powerful antibodies, the strongest of which neutralizes 91% of HIV strains, more than any AIDS antibody yet discovered.
And here’s CNN’s view:
After years of disappointment, researchers have finally found a potential basis for an HIV vaccine. Scientists at the National Institute of Allergy and Infectious Diseases say they have discovered three human antibodies that neutralize more than 90 percent of the current circulating HIV-1 strains.
The work is scientifically very interesting, and indeed is a major advance in our understanding of how antibodies just might prevent this infection after all.
But on a practical level, I’ve learned that every HIV vaccine research “breakthrough” or prediction needs to be taken with more than a few grains of salt.
Including this memorable one way back in 1984:
Finding the cause of AIDS will not necessarily lead to any treatment of the disease soon, nor will it necessarily result in a method of prevention. But the finding led the American researchers to express the hope that a vaccine would be developed and ready for testing ”in about two years.”
As the scientists involved in these current Science studies are quick to say, an actual effective vaccine for use in the clinic based on the results of these observations is many (many) years of hard work away.
Which is (for now at least) pretty much how we should view any HIV vaccine news in the mainstream media.
July 6th, 2010
Torrid Tuesday
 Some ID/HIV-related items for a sweltering summer day:
Some ID/HIV-related items for a sweltering summer day:
- Are the AIDS Drug Assistance Programs (ADAPs) in trouble? Certainly in some states they are, and this interview gives additional perspective. But I wonder — how much of this is HIV-specific, and how much is just the ongoing lousy economy. In other words, are other government-funded programs comparably stressed?
- Headline: Men on ED Drugs Get More STDs. But read the fine print in the actual study — the risk was greater the year before they received the prescription as well. Some serious confounding going on here, I bet.
- XMRV and Chronic Fatigue Syndrome? CDC says no; NIH says … maybe. You decide.
- Great review of the (nearly empty) HIV drug development pipeline here — and I’m not saying that just because I make a small appearance towards the end!
- You mean taking the catheter out promptly doesn’t improve the prognosis of patients with candidemia? Granted, it’s only one study, but it’s fairly large (n=842), and raises the question — how many other “rules” of ID consultation are not data driven? I suspect a lot.
- Uh-oh … first coyotes, and now this? A rabid bat in my home town? Perhaps I’ll move to Canada.
Stay cool, it’s hot out there!
July 1st, 2010
RFA-AI-10-009, HIV Cure, and “Berlin Patient” Update
 Interesting “RFA” (Request for Application, #RFA-AI-10-009) from Bethesda:
Interesting “RFA” (Request for Application, #RFA-AI-10-009) from Bethesda:
The National Institute of Allergy and Infectious Diseases (NIAID) and the National Institute of Mental Health (NIMH), National Institutes of Health (NIH), encourage grant applications from institutions/organizations to address the problem of HIV-1 persistence in HIV-1-infected persons treated with suppressive antiretroviral drug regimens… The goal of this initiative is to expand the knowledge base on HIV-1 latency and persistence so that eradication strategies can be designed, developed and evaluated.
In other words, research directed at a cure. If there’s any doubt about what the ultimate goal of the grant is, the title of the RFA is “Martin Delaney Collaboratory: Towards an HIV-1 Cure (U19)”, named in honor of the influential patient advocate who died last year.
I’ve written before about the “Berlin Patient” (here and here, for example) who, after receiving a bone marrow transplant from a donor homozygous for the delta-32 CCR5 mutation, was able to stop all antivirals without experiencing virologic rebound. The latest on him from the “cure” perspective is that even Bob Siliciano’s lab can’t find virus in the guy:
As of today, [the Berlin patient] remains healthy, and his HIV levels are undetectable … Even samples sent to Siliciano’s lab and other U.S. facilities that have the most sensitive assays have come up empty. Says Siliciano: “I think he’s cured.”
From single case report — one that began with an obscure research poster at the 2008 Retrovirus conference — to a complete cure of HIV, this case must be showing us the way to eradicate the virus.
And if you’d asked me ten years ago which I thought we’d see first, a truly effective HIV vaccine or a legitimate pathway to an HIV cure, I’d definitely had said the vaccine.
Now I’m not so sure …
June 23rd, 2010
Combined HIV Antibody/Antigen Test Approved
 From the FDA:
From the FDA:
The U.S. Food and Drug Administration today approved the first assay to detect both antigen and antibodies to Human Immunodeficiency Virus (HIV)… The highly sensitive assay is intended to be used as an aid in the diagnosis of HIV-1/HIV-2 infection, including acute or primary HIV-1 infection. Since it actually detects the HIV-1 virus (specifically the p24 antigen) in addition to antibodies to HIV, the ARCHITECT HIV Ag/Ab Combo assay can be used to diagnose HIV infection prior to the emergence of antibodies.
This is definitely progress, as it seems fairly obvious that combined antibody/antigen detection is how we should optimally be diagnosing HIV infection.
The fact remains that despite the increased sensitivity of antibody testing, some HIV cases are still missed when patients present with recently-acquired infection, too soon after viral transmission to have detectable antibody. One acute case we saw recently had reactive ELISA, but a completely negative Western blot — anecdotally this is becoming an increasingly common “negative” HIV test since ELISA has cranked up the sensitivity so much.
The two settings where this new test might be particularly useful are in high-prevalence populations (where the incidence is higher) and when people seek testing soon after a risky exposure.
Remaining questions include the cost of the new assay (both in literal terms and in lab personel resources), the predictive value of a reactive result in low risk settings, and how the p24 antigen here compares in sensitivity with HIV RNA measurement (our usual way of detecting acute HIV). On the last issue, at least one past study suggested that p24 was less sensitive than HIV RNA, but that was a long time ago.
(FYI, June 27 is National HIV Testing Day. Apologies for the grammatical error — “a HIV test” — in the above image, I love the poster.)
June 16th, 2010
Another HIV Drug Development Program Bows Out
 Last month, Avexa announced that they will not be going forward with their development of the investigational NRTI apricitabine.
Last month, Avexa announced that they will not be going forward with their development of the investigational NRTI apricitabine.
Now Myriad says its program to develop bevirimat is closing as well.
The problems with these drugs — twice daily dosing with apricitabine, formulation and mixed responses with bevirimat — are not the real story here, since arguably we have overcome these hurdles with investigational drugs before.
Nope, the real issue here is that the challenge of HIV drug development has become staggeringly high due to the very success of our current therapies. Who wants to spend the resources on therapies for drug resistance when hardly anyone fails treatment anymore?
Which leaves me wondering: what are we going to do with that small population of patients who have literally no drug options? The group with NRTI, NNRTI, PI, enfuvirtide, and integrase resistance who have dual-mixed tropic virus?
Time for “orphan drug” status for investigational HIV drugs that meet this need? Certainly some sort a national collaborative effort would be welcome, as most practices have very few of these unfortunate patients.
June 11th, 2010
Plays at the (Culture) Plate
 Some quick ID/HIV/other thoughts while we marvel in all that is Strasburgian:
Some quick ID/HIV/other thoughts while we marvel in all that is Strasburgian:
- Did you know that HIV medication adherence improves over time? So much for “pill fatigue.” By the way, this anecdotally fits with my experience as well. And right now, the biggest reason for patients’ stopping their HIV meds is financial, usually due to loss of or change in insurance.
- This BMJ paper showing that getting an antibiotic in the primary care setting increases the risk of resistance in the person taking the antibiotic should be distributed to every outpatient practice in the universe — and then be prominently posted in waiting rooms.
- More on the underutilized zoster vaccine in the NY Times. Again, from my admittedly warped perspective as an ID doctor — one I’ve already confessed to here — this is one adult immunization not to skip! Biggest barrier? Who’s going to pay the $300 or so for the vaccine. For an example of ironic journalistic juxtaposition, an adjacent piece cites the hundreds (or even thousands) of dollars people might spend on the health of their pet.
- Over on KevinMD, here is a list of suggestions for how to talk to patients while using an electronic medical record. Some good advice — but the bottom line is that something is lost when our attention is split between the patient and the glowing screen. Just think about trying to get your kid’s attention while he/she is on-line. Or vice-versa.
- Want to increase the nasty (as in raunchy) comments auto-posted on your blog? Easy, just include a few choice words in your post’s title. Let’s just say that our spam filter has been busier than usual recently.
Hey, peak Lyme season is here — watch out everyone, hungry ticks are everywhere!
June 2nd, 2010
Screening for Anal Cancer and the World’s Worst Job
 In Journal Watch AIDS Clinical Care, we published a simple case: Clinically stable HIV+ gay man, on HIV treatment; anal pap comes back with “atypical squamous cells of undetermined significance” (ASCUS).
In Journal Watch AIDS Clinical Care, we published a simple case: Clinically stable HIV+ gay man, on HIV treatment; anal pap comes back with “atypical squamous cells of undetermined significance” (ASCUS).
What to do with this result? Two experts weighed in, Howard Libman and Joel Gallant. In Howard’s thoughtful response, he acknowledges the limitations of the data thus far, but said he would refer the patient for high-resolution anoscopy (HRA) and biopsy — which is what most of our readers said as well.
But Joel acknowledges that, despite an institutional protocol to refer all such patients, he wavers a bit for those with ASCUS:
I have been bending the rules in patients with ASCUS and monitoring them with yearly Pap smears rather than referring them for HRA. I do this with the understanding that the Pap smear provides an imprecise measurement of the true grade of dysplasia and that those with ASCUS could have higher grades on biopsy. However, I have to weigh that risk with the fact that my patients don’t enjoy going through HRA, biopsy, and ablation, that the parallels between anal and cervical dysplasia aren’t perfect, and that the protocols around anal Pap smear are written without much evidence backing them up.
It’s safe to say that we don’t really know yet what to do — not in this situation, nor in multiple other scenarios involving anal cancer screening.
Just a few questions to ponder: How frequently (if at all) should anal paps be done? If the sensitivity is so poor, why not refer all gay men for HRA/biopsy, skip the pap? Or should it be limited to those with a history of condyloma? (Read this concerning paper.) Or should those patients go right to biopsy? Should HPV testing be done in all patients? What anal cancer screening should be done in HIV-infected women? Or should this just be done in women with cervical disease?
If the evidence were stronger, clearly the Primary Care and OI Prevention Guidelines would recommend a standard screening protocol.
But until then, one hear’s the voice of Joel Palefsky — owner of the “World’s Worst Science Job” — as he highlights the benefits of preventing a common, potentially life-threatening and disfiguring cancer. In response to his (and other’s) work, a diverse range of practitioners out there (PCPs, ID docs, gynecologists, dermatologists, surgeons) now offer high-resolution anoscopy with biopsy for this indication.
But is screening for cancer always the right thing to do?
May 23rd, 2010
Dengue in the News … Again
The recent dengue cases acquired in Florida prompted me to think of two things.
First, is this really a surprise? Dengue has become increasingly common in the Caribbean, the mosquitoes that transmit the virus are widespread in the United States, and it’s not as if there’s some sort of microbiologically (if that’s a word) impermeable barrier between the islands in the Caribbean and our southernmost states:
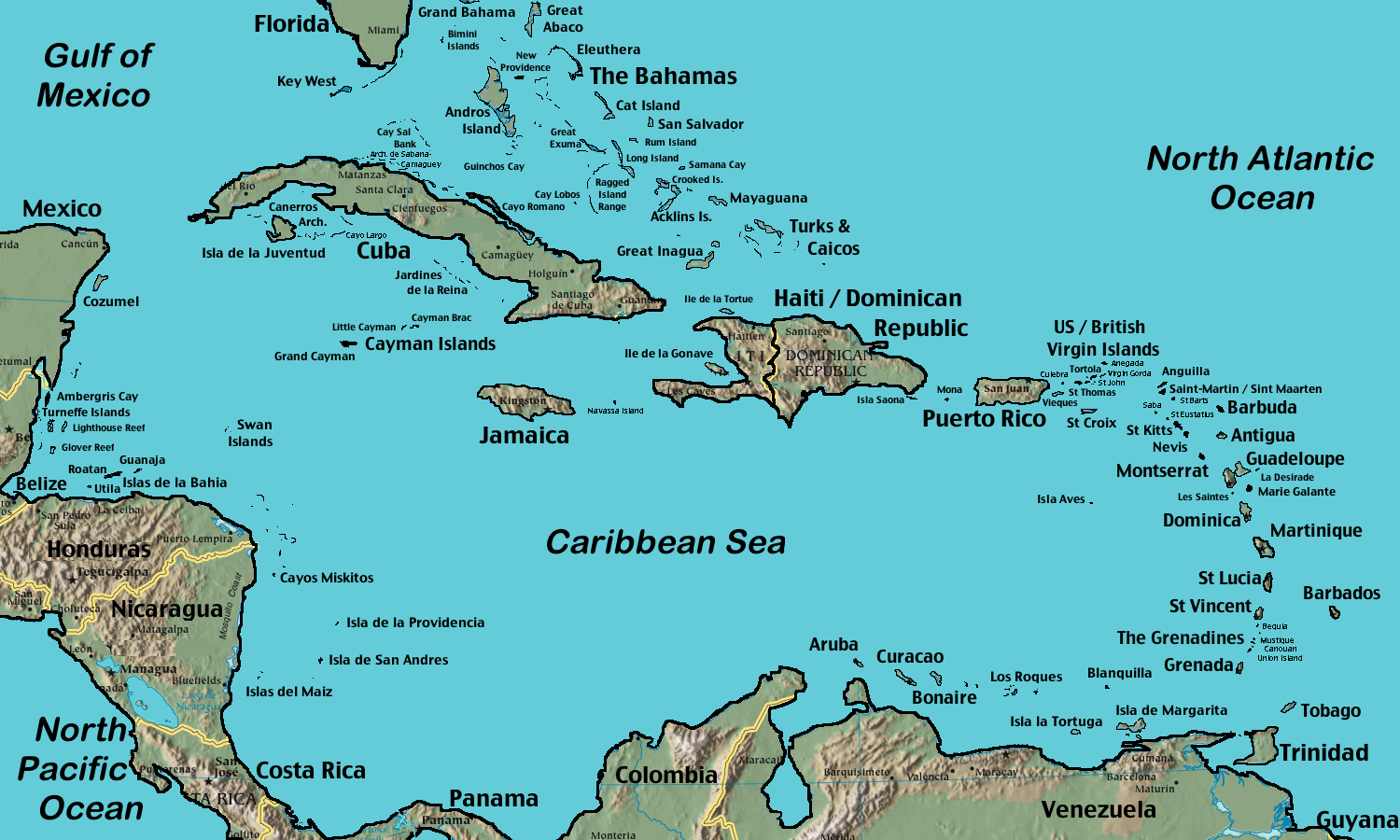
Add a dose of climate change (not mentioned in the MMWR editorial — just a hunch), and bingo, the perfect recipe for tropical diseases in the Continental 48.
Still, many kudos to the astute ID doctor in Rochester NY who made the diagnosis:
During this third visit [to her primary care doctor], a consulting infectious-disease specialist raised the possibility of dengue infection, despite no recent travel by the patient to a known dengue-endemic area.
Hey, this is why they pay us the big bucks!
The second thing I thought about was another time dengue was the headline in MMWR. Anyone remember the really big news in MMWR when the lead was “Dengue type 4 infections in U.S. travelers to the Caribbean”?

 The Lancet has
The Lancet has 