An ongoing dialogue on HIV/AIDS, infectious diseases,
May 11th, 2024
Just in Time for Mother’s Day, Some Admiration and Gratitude
 As I’ve written here before, I’m in awe of my mother — a smart woman who doesn’t celebrate Mother’s Day. So in place of celebrating, I’m going to use the holiday anyway as an excuse to share an event that highlights her strengths and resourcefulness. It has an ID theme eventually, so stick around to the end.
As I’ve written here before, I’m in awe of my mother — a smart woman who doesn’t celebrate Mother’s Day. So in place of celebrating, I’m going to use the holiday anyway as an excuse to share an event that highlights her strengths and resourcefulness. It has an ID theme eventually, so stick around to the end.
By way of background, my mother regularly uses New York City’s public transportation system, both the subways and buses. No big deal, one might think, so do umpteen million others. But the reason I mention it is because my mother is old. How old? Just look at me, and do the math for a good approximation. To be specific, she’s about to start her tenth decade.
Plus, she’s been living on her own since my father died, and continues to take care of pretty much everything in her life. This includes food shopping, cooking for herself and cleaning, and managing her surprisingly busy social and academic life. (She has a recent volunteer gig as a teacher, newsletter editor, and student.) She does these things without making a big deal out of it, so my brother, sister, and I take it for granted sometimes, overlooking how remarkable this independence is.
But a few months ago, I received a call that my mother had fallen while getting on the bus and was receiving care in an emergency room. She had no broken bones — thank goodness — but the fall took a sizable chunk of flesh from her left leg. Someone witnessing the injury thought she might need a tourniquet to control the bleeding.
We’ve all seen what falls can do. And it’s not just to older people — I’ve taken care of a man in his 30s whose life was irreversibly changed after falling from a ladder in his kitchen, striking his head, breaking his ankle, and triggering a series of neurologic and infectious complications that left him permanently impaired.
(I’m terrified of ladders. My wife thinks I’m a wimp, but I know better.)
Of course when it comes to falls, older people are especially vulnerable. Neuropathy, muscle weakness, vestibular instability, visual impairment, osteoporosis, and arthritis all come together to make falls way more common — and treacherous — in us as we age. The falls cause physical and psychological trauma that can profoundly weaken a person, leading to an amplifying cycle of debilitation and complications and dependency.
Think how many times you have heard, “He was OK before the fall …” or “Ever since the hip fracture, she’s never been the same.” Shudder.
In my conversations with my mother after the event, the tone of fragility in her voice was one I had never heard. Plus, she was barely leaving her apartment.
But rally she did:
- She openly shared with her family and friends how hard this process was — not an easy disclosure for a person generally independent and hesitant to reach out for help.
- From a skilled and incredibly kind plastic surgeon (Thank you, Dr. Schwartz!), she learned how to monitor and dress her sizable wound each day. I watched her do it, and think she could have had a career as a wound care nurse had she not been a journalist. What talent!
- She gradually increased her ability to get around again, first going for short walks outside when the weather was good, then starting to shop again on her own. She’s now back on public transportation.
- She managed to take a week of levofloxacin without destroying her tendons.
So we finally come to the ID part of this post. When the healing seemed to be slowing, with increased drainage, her plastic surgeon sent a wound culture, results of which he shared with me in this screen shot:
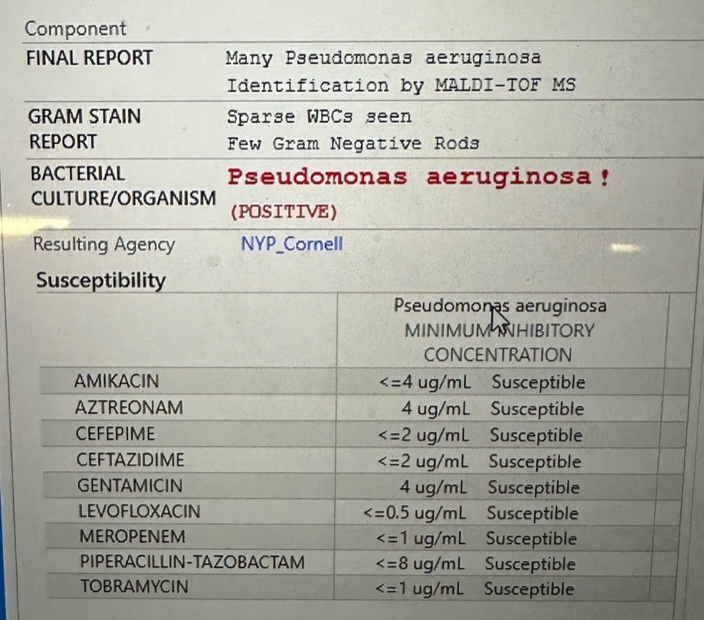
“Unless you have another thought, I’m going to start levofloxacin,” he wrote to me. Yes indeed, good plan — it was a superinfection of this widely open wound. Perhaps it was selected by the previous course of cephalexin she took. Or maybe it was just a “gift” from the flora of the New York City streets.
Ever curious, my mother had two questions, my answers in brackets:
Is this the infamous “flesh eating” bacteria? [No, that’s most often strep.] If this is a pseudo (meaning fake) monas, what’s the real monas like? [I have no idea.]
These are excellent questions, especially the second one.
I’m happy to report that with local care, and antibiotics, and time, the wound has ever-so-slowly healed. She’s “graduated” (her term) to just using a small bandage. No more visits to Dr. Schwartz.
So Happy Mother’s Day, Mom — glad you’re getting better, you did amazingly well. And be careful on those city buses!
April 26th, 2024
Hey, Insurance Companies and Pharmacies — Stop Messing Around with the Price of Cheap Generic Drugs

Photo by Markus Winkler on Unsplash
If you’re practicing medicine these days, you’ve likely experienced some version of this painfully annoying scenario.
- You prescribe a generic medication, one that’s inexpensive.
- Your patient goes to the pharmacy, and the pharmacist says that it requires a prior approval.
- They leave without getting their meds.
Here’s a recent example from one of my patients (details changed to protect confidentiality):
A 74-year-old man goes to the emergency room with facial cellulitis. I am called by the emergency room clinician, who wants to prescribe cephalexin plus trimethoprim-sulfamethoxazole. (He said Keflex and Bactrim because he just can’t quit the trade names.) I suggest linezolid, which has been available as a generic for years. A 10-day treatment course of linezolid is prescribed by the ER doc and sent electronically to his pharmacy. When my patient gets there — face all swollen, in pain — he is told that the prescription “requires a prior approval.” He leaves with nothing. The emergency room doctor is off their shift, so the patient’s daughter pages me to get the prior approval done.
Why is this insane? Because linezolid, which used to require prior approvals due to high cost, is now good value without insurance coverage. Requiring a prior approval makes zero sense when you can get the generic for such low out-of-pocket costs.
GoodRx says that with one of their coupons, the price could be as low as around $35 for 10 days.
CostPlus drugs charges around the same for 15 days, and they’ll mail it to you.
But you’ll note on the GoodRx site that there’s quite the range — the “retail prices” can be as high as $3500! And one of the big pharmacies still charges over $800.
These shenanigans have been going on for years with drugs far more commonly prescribed than linezolid. From a 2017 article in The New York Times:
In an era when drug prices have ignited public outrage and insurers are requiring consumers to shoulder more of the costs, people are shocked to discover they can sometimes get better deals than their own insurers. Behind the seemingly simple act of buying a bottle of pills, a host of players — drug companies, pharmacies, insurers and pharmacy benefit managers — are taking a cut of the profits, even as consumers are left to fend for themselves, critics say.
Here’s what I think is happening with linezolid, but who knows. The payers (or their henchmen the Pharmacy Benefit Managers) require a prior approval because linezolid used to be so expensive, and they haven’t updated their policy. And pharmacies (who may be owned by the same mega-company), try to get patients to use their insurance to get the higher price covered.
But this strategy can clearly be harmful to patients. And it’s dishonest almost to the point of being fraud.
I confess that I don’t know with 100% certainty why some payers and pharmacies require a prior approval for linezolid, but I very much do know what the ethical thing to do would be. The beleaguered, overworked frontline pharmacist — and I am very sympathetic to their plight! — would, on seeing that an inexpensive generic drug requires a prior approval, say the following:
I’m sorry, Mr. Smith. Your insurance company says you need a prior approval for this drug before I can fill your prescription here. But if I were you, I’d ask your doctor to send a prescription to pharmacy X, Y, or Z, where they will give you the generic for way less money if you don’t use your insurance. For that matter, your out-of-pocket payments there may be even less than your co-pay if you fill your prescription here with your insurance.
There are a lot of things to be proud of when you’re a U.S. citizen. But this crazy healthcare system we have sure isn’t one of them.
April 8th, 2024
The Rise and Fall of Paxlovid
It’s been quite the ride for our “preferred” outpatient therapy for COVID-19, nirmatrelvir with ritonavir — much better known as Paxlovid, so allow me the license to use the licensed name.
Let’s recap the astonishing success and now failure of this intervention (some dates approximate):
- December 2021, the FDA issued an Emergency Use Authorization for Paxlovid: Action is based on the efficacy shown in the EPIC-HR study of high-risk outpatients with COVID-19. Compared to those receiving placebo, Paxlovid-treated participants had an 89% reduction in risk of hospitalization or death. Exciting times.
- Early 2022, that annoying rebound thing. No, it’s never been quite clear whether Paxlovid caused rebounds, or just didn’t prevent them, or whether it just happened in those people for whom COVID-19 illness lasted longer than the treatment’s 5 days (a large group!), but regardless — it was a major disincentive to clinicians and providers alike.
- December 2022, the protocol for the EPIC-SR in “standard risk” outpatients was amended to increase the sample size. Though an interim analysis suggested such patients would benefit from treatment, the change in sample size signaled that such a benefit observed in this analysis might be small — or nonexistent.
- August 2023, the negative results of EPIC-SR were posted on clinicaltrials.gov. Yes, these results have been in the public domain since last summer.
- Last week, the disappointing EPIC-SR results appeared in the New England Journal of Medicine. This is the primary endpoint (from the Research Summary):
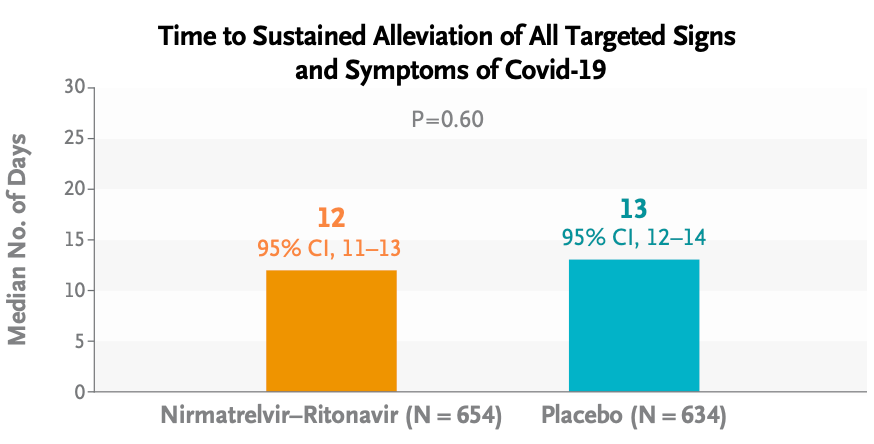
Oh well.
It wasn’t all bad news for treatment: Risks of severe outcomes (hospitalizations, ICU admissions, or deaths) were very low overall — hooray! — and numerically lower in the Paxlovid group; medical visits were significantly lower as well. However, as anyone who has taken Paxlovid can attest, the taste alone could have made study participants aware that they were already on treatment, hence discouraging them from seeking further evaluations.
What about rebounds? From these limited data, it appeared there was no difference:
By day 14, viral load rebound had occurred in 4.3% of the participants in the nirmatrelvir–ritonavir group and 4.1% of those in the placebo group; symptom rebound occurred in 11.4% and 16.1%, respectively, and symptom and viral load rebound together occurred in 1.2% and 0.5%, respectively.
So where does that leave us right now? There’s no doubt that the EPIC-SR data further confirm that this treatment has many limitations. Here’s a teaching slide I recently made:

(If you’re wondering about the picture of the Fab Four, I can’t reference anything based in Liverpool without citing two of its spectacular exports — the Beatles and the University of Liverpool drug interactions checker!)
But before we completely abandon Paxlovid for outpatient use, let’s remember that our options for outpatient treatment of COVID-19 remain highly limited. Furthermore, people at high risk for adverse outcomes still require hospitalization and still die from this infection — as they do from other viral respiratory illnesses. As a result, based on EPIC-HR, these EPIC-SR data, and the observational studies, I’d still recommend treatment for this very high-risk group.
For others? Allow me to quote (with his permission) my smart colleague Dr. Athe Tsibris, a virologist who sent me this email after his own unfavorable experience with treatment last fall — the subject line read “Boo Paxlovid”:
Hi Paul,
Having just gone through Paxlovid rebound with my 1st covid infection (I held out as long as I could!), I’d like to share an entirely unsolicited observation and two rants:
1. The dose and/or duration is clearly wrong. Started within 48hrs of symptoms and rebounded – documented with daily antigen tests – harder than then original infection!
2. The company line of 2% incidence of rebound is laughable
I would not take Paxlovid again. I understand the limitations of human psychology, but for someone very low risk like myself, I don’t know how hard I’d push it on similar patients
Athe
For low-risk people, I totally get it.
Meanwhile, the gargantuan PANORAMIC study of Paxlovid in outpatients has closed to enrollment, with data collection continuing until September. Will this study provide any further evidence of the benefits or risks of treatment?
Regardless of the results, I do hope that the appetite for better treatments for COVID-19 remains, as this virus isn’t going anywhere.
March 27th, 2024
Think Again Before Sending Your Patient Home on Intravenous Vancomycin
Chemical structure of vancomycin — you knew that, right?
I took care of a patient many years ago with MRSA. The severity of the infection required a prolonged treatment course, and vancomycin was the default option. Cripes, it was the only option. He ultimately was discharged on home IV therapy, and as usual we had a plan to monitor his renal function and vancomycin levels weekly.
The twist was that his wife was a nephrologist. Weekly blood tests weren’t enough; she wanted twice-weekly labs. She scrutinized all the results with meticulous intensity, plotting them on a spreadsheet so we could stop the drug “at the first hint of trouble.”
When we spoke, I was strictly instructed to dose the drug so that the trough concentrations never exceeded 10 mcg/mL. “Vancomycin is poison,” she said. A memorable quote!
(The patient did fine, low troughs notwithstanding. It was a team effort! And yes, I changed some details for patient confidentiality.)
Fast forward to today, with our target vancomycin troughs between 15–20 — or with area under the curve monitoring — and there’s no doubt that some of this nephrologist’s heightened concerns about nephrotoxicity have come to pass. Any doubts that vancomycin has nephrotoxicity have been dispelled by study after study showing a clear dose relationship between drug exposure and renal injury. It’s particularly problematic in older patients or those with other risk factors for renal disease.
The problems with vancomycin are much on my mind, as a trio of great first-year ID fellows just reviewed a particularly difficult outpatient vancomycin case. They cited one study of 130 patients on outpatient vancomycin where a whopping 28% developed some sort of renal injury. Risk factors were higher cumulative dose, a longer duration of therapy, underlying kidney disease, and use of other nephrotoxic meds. No surprise, there are a lot of studies with similar findings.
Of course, when I was treating my patient way back when, options for MRSA treatment were highly limited. What’s changed today is that we now have several alternatives to vancomycin — alternatives that are better in many important ways. As a result, it’s time we demote vancomycin substantially when discharging patients who need ongoing antibiotic therapy.
Here’s why:
- Other options are less toxic. Daptomycin, linezolid, ceftaroline, trimethoprim-sulfamethoxazole, and doxycycline aren’t perfect; all drugs have side effects, after all. But in OPAT land, vancomycin troubles greatly exceed issues caused by these other antimicrobials. For the comparison between vancomycin and daptomycin, our astute ID fellows identified two typical studies (one from 2014, the other 2018) showing significantly fewer adverse events for daptomycin. Bottom line — it’s safer!
- Monitoring vancomycin levels is hugely resource intensive. Area under the curve monitoring, now the preferred method for people with stable renal function, requires the input of a pharmacist, and isn’t available in many settings. Targeting the optimal trough concentrations, especially in people with changing renal function, is an obstacle course that could lead to an awful lot of falls, sometimes with no safety net. It’s never quite right!
- What’s a “trough,” anyway? If you haven’t had a “trough” vancomycin level checked at the wrong time — the error disclosed to you by your observant patient, their caretaker, or your home care service — you haven’t yet experienced the deep frustrations of monitoring home IV vancomycin. On some extreme misfires, a peak level is drawn accidentally, scaring everyone, but providing essentially useless data that needs to be repeated as a “true trough.” Fun times.
- Oral therapy can be substituted for intravenous in a high proportion of patients. Cue Dr. Brad Spellberg here for his very convincing, “Busting 75 Years of ID Myth” talk!
- Who’s paying for all this careful monitoring? As I’ve written before, home IV therapy is the classic hot potato clinical service, terribly reimbursed, and vancomycin is definitely the most common culprit in adding to this burden. For us physicians, the measures of our productivity still predominantly come from face-to-face patient visits and procedures — not calling skilled nursing facilities to chase down creatinine results and vancomycin levels.
- Prolonged infusion times make patients prisoners of vancomycin administration. Each dose takes more than an hour — sometimes much longer. This is true for all home vancomycin recipients, but the ones I truly feel bad for are those who require every 8-hour vancomycin dosing — they are spending what must feel like most of their waking hours watching IV vancomycin slowly infuse. With its once-daily dosing and an administration time lasting just a few minutes, it’s no wonder patient satisfaction is significantly higher with daptomycin than vancomycin. I bet they’d be even higher with dalbavancin or oritivancin prior to discharge, though unfortunately the clinical research on using these drugs for severe MRSA infections is limited.
- There’s no longer a cost advantage. Daptomycin and linezolid prices have (at last) dropped substantially from their stratospheric peaks in their brand-name days. Trimethoprim sulfamethoxazole and doxycycline are even less expensive. Additionally, alternatives to vancomycin spare the additional costs of drug level monitoring and drug toxicity.
Conspicuously absent from the above list are citations about treatment effectiveness. If we knew that vancomycin had superior efficacy to alternatives for home IV therapy, then one could justify all the hassle. But if such studies exist, I’m certainly not familiar with them.
So prior to discharging that patient on home vancomycin, think very carefully about safer and more convenient alternatives. There is almost always going to be a better option.
March 13th, 2024
CROI 2024 Denver: Really Rapid Review
 One of the most rewarding things about social media in medicine is tapping into the minds of other smart people in your field, especially people you can’t otherwise interact with on a day-to-day basis. When that person is someone like Dr. Sébastien Poulin — a funny and indefatigable ID/HIV doctor from Montreal — it’s especially worthwhile.
One of the most rewarding things about social media in medicine is tapping into the minds of other smart people in your field, especially people you can’t otherwise interact with on a day-to-day basis. When that person is someone like Dr. Sébastien Poulin — a funny and indefatigable ID/HIV doctor from Montreal — it’s especially worthwhile.
Over the past few years, he’s taken the time to scan the entire program of an upcoming ID/HIV conference, selecting his highlights. With the caveat that no one’s highlights will be exactly like anyone else’s, these reviews have nonetheless provided a framework for going to an upcoming meeting that I’ve learned from, valued, and greatly enjoyed.
Well, this year Dr. Poulin could not make the Conference on Retroviruses and Opportunistic Infections (CROI) 2024, which took place in Denver last week. So with a nod to him — get well soon! — here’s a Really Rapid Review™ of some of the studies from this year’s meeting that I found particularly interesting.
It’s the first time CROI has been in Denver since 2006 and, while no one presented a viable strategy for HIV cure or an effective HIV vaccine, we saw plenty of interesting studies. Here’s a sampling, roughly ordered by treatment, complications, and prevention.
After virologic suppression, long-acting injectable cabotegravir and rilpivirine (CAB-RPV) was superior to oral antiretroviral therapy (ART) in those who struggle with ART adherence. So many good things to say about this study — it was randomized, it used economic incentives successfully, it included a population rarely included in clinical trials. And here’s an irony: the net benefits of using CAB-RPV on a per-person basis are likely to be much greater in this study group than in those for whom the treatment is FDA approved, who are already doing well on oral ART. Think about that for a moment.
CAB-RPV injected every 2 months was noninferior to continued oral ART in Africa. Conducted in South Africa, Uganda, and Kenya, the study demonstrated that this treatment would be effective even with infrequent (every 24 months) viral load monitoring and, more remarkably, with a high proportion harboring non-nucleoside reverse transcriptase inhibitor (NNRTI; 10%) and integrase strand transfer inhibitor (INSTI; 8%) mutations on proviral genotypes done on baseline samples. (I still would not recommend this regimen for people with known resistance!) Two of 256 CAB-RPV recipients developed integrase resistance.
Once-weekly lenacapavir and islatravir maintained virologic suppression. The weekly dose of islatravir was 2 mg and did not lower total lymphocyte or CD4 cell counts. This is the first once-weekly oral ART regimen with demonstrated efficacy, with phase 3 studies planned.
Other once-weekly oral agents are in development. These include an integrase inhibitor, an NNRTI, and a nucleoside reverse transcriptase translocation inhibitor (NRTTI).
In a study conducted in Haiti, switching people on a second-line boosted protease inhibitor (PI)-containing regimen to BIC/FTC/TAF was highly effective. Such individuals are known to have high rates of nucleoside reverse-transcriptase inhibitors (NRTI) resistance, so this reinforces the results of the 2SD study, which showed the safety of the boosted PI to second-generation integrase switch in maintaining virologic suppression — even without knowledge of this resistance. Proviral DNA resistance testing is planned. Remarkable that the primary investigators on site in Haiti could conduct this study in a setting with such extreme civil unrest, a heroic task. (Disclosure: I am a co-investigator.)
In an open-label, phase 2 study, daily oral bictegravir plus lenacapavir was compared to a continued complex regimen, with comparable rates of virologic efficacy. While they were given separately in this phase 2 study, a co-formulation of BIC/LEN is currently in phase 3 trials.
Twice-daily BIC/FTC/TAF achieved comparable virologic suppression to TDF/3TC plus DTG twice daily in HIV-related tuberculosis. Impressively, virologic suppression was observed in 97% of participants in both arms. After the presentation, some raised concerns about the unnecessary doubling of the TAF/FTC dose in the BIC/FTC/TAF group, but these agents are very unlikely to cause toxicity even with this increased exposure — and none was observed.
In a large (N=1362) observational study, the effectiveness of CAB-RPV in clinical practice was similar to clinical trials. Confirmed virologic failure occurred in 2% of individuals; a second study (N=278) reported an incidence of 0.9%. In one single-site report, 4% (3 out 75 patients) experienced this negative outcome and are now on PI-based regimens, with a suggestion that irregular injection practices at an independent infusion center may have contributed. Treatment failure with resistance is the most dreaded outcome of this CAB-RPV regimen, so it’s good to have these “real world”* data.
(*Ouch. Some people hate that term. I find it mildly annoying, preferring “in clinical practice”, which my friend Dr. Eric Daar hates. Oh well. But “real world” is so widely used these days it’s hard to avoid — so I succumbed and used it.)
A case series demonstrated the effectiveness of combining long-acting cabotegravir plus lenacapavir. The most commonly cited reason for using cabotegravir without rilpivirine was baseline RPV resistance — which is especially likely in people with long-term HIV and prior treatment failure, and is a major issue globally. From a practical standpoint, cabotegravir alone (without RPV) for HIV treatment is not FDA approved — only for prevention — which means that clinicians must discard the RPV when obtaining it for treatment and used in this fashion.
Ward 86 in San Francisco again reported high rates of viral suppression for people with viremia who were treated with CAB-RPV. Out of 59 such patients followed to week 48, 81% (48/59) remained on LA-CAB/RPV and were virally suppressed; an additional 7 patients were on alternative ART and suppressed. Only 3 of 59 (3%) experienced virologic failure with resistance. These are some of the core data that motivated the change to the IAS-USA treatment guidelines, discussed here previously.
Several studies (abstracts #117-121) with broadly neutralizing antibodies (bNAbs) demonstrated that we’re still far from seeing these agents as part of viable ART strategies. Issues remain the complex, slow, and expensive test for resistance; a high proportion of people with pre-existing resistance once the test is done; intravenous administration (for some bNAbs); and, even if levels achieved are adequate with a susceptible virus, still a higher rate of failure than we see with standard ART. On the flip side, bNAbs may offer the first chance at twice-yearly therapy (with lenacapavir); updated results from that study were presented in a small number of patients.
Two years after a programmatic switch to tenofovir/3TC/dolutegravir (TLD), virologic failure with DTG resistance was observed, but uncommon. Not surprisingly, it was more likely in those who were viremic at switch — individuals who not only struggle with adherence but also are more likely to harbor NRTI resistance mutations.
A separate prospective cohort study showed that people with treatment failure after switching to TLD had low tenofovir diphosphate levels. Levels were particularly low in those with failure on boosted PI regimens prior to the TLD switch, confirming that suboptimal adherence continued to be a problem for people on failing therapy.
Detection of resistance mutations by proviral (“archive”) genotypes over time is highly variable. It’s not that the mutations come and go — it’s that the sampling process may or may not detect them. The take-home message is that this test has a strong predictive value positive for detected mutations, but negatives should be viewed with appropriate caution.
How effective was lenacapavir in patients with no other fully active drugs? In this analysis from the CAPELLA study of highly treatment-experienced patients, 12 participants had zero fully active background agents when treated with lenacapavir. Regardless, 8 of 12 still achieved and maintained virologic suppression. While such a strategy isn’t recommended (always best to use active drugs in combination), it may be necessary in certain individuals under extreme resistance situations. Results remind me of ACTG 364*, when two NRTIs plus efavirenz — another potent but relatively low-resistance barrier drug — still suppressed viremia in 62% despite extensive baseline NRTI resistance.
(*How’s that for a blast from the past?)
In a randomized clinical trial of hepatitis B non-responders, the HepB-CpG vaccine (HEPLISAV-B) was superior to the standard recombinant vaccine in inducing a seroprotective response. Both two and three doses of the vaccine achieved this favorable result. This is the kind of study that should lead to a change in vaccine guidelines.
In patients with positive hepatitis B core antibodies, switching to a two-drug antiretroviral regimen without tenofovir was not associated with transaminase elevation. This applied even to the 118 individuals not on 3TC or FTC; the study mixed those with and without HBSAb. A second study, by contrast, demonstrated hepatitis B reactivation in 1 of 7 patients with isolated anti-core antibody (no surface antibody) when switched to a non-tenofovir and non-3TC/FTC regimen. A take-home message from the various studies to date? Those with isolated anti-HB core antibodies should, if possible, remain on anti-hepatitis B-containing ART; switches to regimens without tenofovir or FTC/3TC should be monitored for hepatitis B reactivation.
Simultaneous initiation of HIV and hepatitis C virus (HCV) treatment was highly effective. The regimens used were BIC/FTC/TAF and SOF/VEL for HIV and HCV, respectively. Out of 128 patients enrolled (52 HIV treatment-naive), all achieved HIV viral suppression, and 98.4% (126/128) had HCV cure by sustained virological response (SVR) 24 assessment. Imagine trying to do something like this in the early ART and interferon for HCV eras — it is extraordinary how far we have come with HIV and HCV treatment!
The final results of the DOXYVAC study were presented — and the meningitis B vaccine just missed demonstrating significant protection against gonorrhea. This randomized trial looked at both doxy PEP and the meningitis B vaccine in a factorial design, and we learned at last year’s CROI that doxycycline postexposure prophylaxis (doxy-PEP) intervention was protective. These updated results showed the incidence of gonorrhea in the vaccine arm was numerically lower than no vaccine (58.3 vs 77.1/100 person-years, respectively), for a hazard ratio of 0.78 (95% CI 0.6-1.01)*. When a result is this close, the most conservative conclusion is that “a small benefit cannot be excluded.” Even if that benefit is real, a better gonorrhea vaccine would be of great use!
(*Time to quote Maxwell Smart.)
In San Francisco, a policy of recommending doxy-PEP to men who have sex with men (MSM) and trans women was strongly associated with a decline in the incidence of chlamydia and syphilis. No significant change was observed for gonorrhea. Additional supportive data on doxy-PEP came from a single clinic site and from the open-label extension of the DOXYPEP study. National guidelines are expected soon; they’re already in draft form.
In a VA-based study, prostate cancer was diagnosed at a later stage in men with HIV versus HIV-negative controls. This finding is of particular interest since prostate cancer has been historically one of the few cancers not observed to have a higher incidence in persons with HIV (PWH), strongly suggesting this result represents a screening gap — which, though controversial as a general tool, is still recommended in higher risk men.
Using data from the REPRIEVE trial, investigators reported that risk-prediction tools underestimated cardiovascular event risk in high-income regions only. The effect was particularly strong in women, who experienced about two and a half times more events than predicted; for Black participants 50% more.
In a prospective single-arm clinical study, semaglutide reduced the amount of liver fat by 31% at week 24. Not surprisingly, weight and glucose control also improved. A second analysis from this study demonstrated a reduction in psoas muscle volume, without impairing physical function. These are two of four studies on glucagon-like peptide 1 (GLP-1) agonists at CROI, and my overall sense is that they’re working the same as in people without HIV — with the caveat that “Ozempic face” might be particularly distressing to people who have baseline lipoatrophy. The slide session started with a terrific review by Dr. Todd Brown.
Switching to a doravirine-containing regimen was associated with weight loss. Although it’s not specifically spelled out in the poster, the bulk of this effect most likely arose from switching the whole regimen to TDF/3TC/DOR, as TDF-based regimens are known to have weight-suppressive effects. Since in a previous clinical trial, there was no change in weight in a previously presented randomized trial of switching from BIC/FTC/TAF to DOR/ISL, it seems unlikely that switching just an INSTI to DOR would lead to weight loss — a question that will be answered by an ongoing clinical trial.
Damage to intestinal enterocytes might explain the weight loss and lipid-lowering effects of tenofovir DF. Twelve men on TDF and twelve on TAF underwent gastroscopy, with biopsies from the proximal and distal duodenum. Those on TDF had more histologic abnormalities (flatter villi and deeper crypts), as well as lower levels of certain nutrients absorbed from the proximal duodenum, and higher levels of serum intestinal fatty acid-binding protein, a marker of enterocyte damage. Both groups had evidence of mitochondrial damage on electron microscopy.
Women who switched to an integrase-based regimen during menopause experienced early accelerated increases in waist circumference and body-mass index. Comparison groups included women with HIV who did not switch regimens, and women without HIV — neither experienced these changes.
In rural Uganda and Kenya, a prevention “package” that offered the options of oral preexposure prophylaxis (PrEP), PEP, and cabotegravir greatly increased the uptake of PrEP over standard of care. The effect was huge — a five-fold increase — and led to a significant reduction in HIV transmissions in those offered the package versus usual care.
In a prospective study of PEP, BIC/FTC/TAF was well tolerated with no seroconversions. Study results support previously published data. Given the low incidence of seroconversion in PEP users, we will never have a comparative clinical trial of different ART strategies that demonstrates one approach is better than another; as such, BIC/FTC/TAF seems like an optimal default choice since it’s simple, well tolerated, and has few drug interactions. Time to include this in PEP guidelines, which are in need of updating?
Three of six children with in utero HIV transmission stopped treatment without viral rebound. All mothers received ART during pregnancy, and the babies started treatment within 2 days of birth. Treatment was stopped at a median of 5 years of age, and the duration of remission was reported as 48, 52, and 64 weeks. Among the 3 children who rebounded, one did not do so until week 80 — hence it’s premature to call these kids cured.
So that’s a wrap! Of course it’s hardly comprehensive, so if I left out your favorite study or studies, have at it in the comments.
Here’s a reminder of the big news out of the first Denver CROI in 2006:
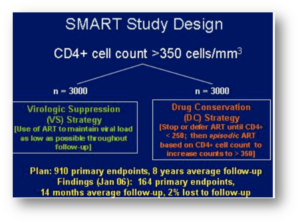
That’s the SMART study of CD4-guided treatment interuption which highlighted the oral presentations. I distinctly remember Dr. Steven Deeks telling me in the hotel lobby, “You’re not surprised at that result, are you? Of course stopping treatment is a bad idea.” As usual, Steve had figured things out long before any of us!
That meeting also featured a trio of drugs destined to change the history of HIV treatment for people with resistant virus — darunavir (TMC-114), etravirine (TMC-125), and raltegravir (MK-0518). In the years to come, many who had never previously achieved viral suppression reached “undetectable” for the first time, and remain so today.
March 2nd, 2024
Just as CROI Gets Ready to Start, an Important Change to the IAS-USA HIV Treatment Guidelines
 One of the top experiences of my ID career has been working with a research group that does HIV disease modeling. The people involved are without exception smart, collaborative, generous, funny, and hard-working — an amazing combination of positive traits.
One of the top experiences of my ID career has been working with a research group that does HIV disease modeling. The people involved are without exception smart, collaborative, generous, funny, and hard-working — an amazing combination of positive traits.
They get this, I believe, from their leader and founder, Dr. Ken Freedberg, who sets a great example. He has been a research mentor and friend of mine for years.
(You might recognize one of his many mentees: Hi, Rochelle Walensky, if you’re reading this!)
Anyway, it’s not often that I disagree with Ken on anything in the HIV treatment area, so it’s been an interesting couple of years as we discussed a strategy that’s been on the cutting edge as a way to manage some of our most challenging cases — namely, the people with HIV who (for a variety of reasons) do not take oral antiretroviral therapy.
As anyone who does HIV care knows, this small fraction of our patient population occupy a disproportionate share of our worries, account for the bulk of hospitalizations and HIV-related deaths, and as a result represent an ironic (and tragic) contrast to the extraordinary success of HIV treatment. It’s unfathomably frustrating on many levels.
The reasons they do not take ART are numerous, and not mutually exclusive — including denial, stigma, mental illness, and substance use disorder — but a glimmer of hope emerged with successful reports of the off-label use of injectable cabotegravir-rilpivirine (CAB-RPV) in a group of people with ongoing viremia who are not taking ART. While these reports first appeared from Ward 86 in San Francisco under the direction of Dr. Monica Gandhi, other studies and anecdotal evidence from clinical treatment sites (including our own) followed.
But back when these reports first emerged, Ken took the position that widespread use of this treatment would require a prospective clinical trial, preferably with a randomization to standard of care, to prove that it works; he furthermore thought that it would not appear in guidelines until such a study took place. I countered that such patients have already proven that they will experience treatment failure if randomized to continue oral therapy, and that such a study would not be necessary to show that injectable ART is the best strategy under these desperate situations.
We bet a nickel. Evidence in photo at the top of this post.
Ken may have gotten his wish. The injectable strategy for people who struggle with adherence got a boost with the announcement of results from the LATITUDE trial, an ACTG study that enrolled people with treatment failure. It provided economic incentives to lower the viral load to undetectable with oral ART, and then randomized them to injectable versus continued oral therapy. The study was stopped early in favor of the injectable ART arm; we’ll see the full results at CROI next week as a late-breaker.
It’s not quite the same population as the one we’ve been discussing (the randomized switch to CAB-RPV in LATITUDE is only after virologic suppression), but it’s close.
Meanwhile, I’ve been energetically pitching to my colleagues on the IAS-USA Guidelines Panel to update our recommendations for use of cabotegravir-rilpivirine for people not able to take oral ART who have advanced HIV disease. The argument is simple: such an intervention can be literally life-saving given the poor prognosis of untreated HIV with low CD4 cell counts, or prior HIV-related complications. Takes us back to the bad old days, pre-1996.
I’m thrilled to report that this revision just appeared in JAMA. Note that this intervention is not for all people who struggle with adherence — just those at the highest risk for HIV disease progression:
When supported by intensive follow-up and case management services, injectable cabotegravir and rilpivirine (CAB- RPV) may be considered for people with viremia who meet the criteria below when no other treatment options are effective due to a patient’s persistent inability to take oral ART:
– Unable to take oral ART consistently despite extensive efforts and clinical support
– High risk of HIV disease progression (CD4 cell count <200/μL or history of AIDS-defining complications)
– Virus susceptible to both CAB and RPV
It will be critical to assess how this treatment does in clinical practice, to monitor for emergent integrase and NNRTI resistance, and to provide the intensive case management services this patient population deserves. And if a prospective study starts employing this strategy, one that does not involve a randomization to continued oral ART, all the better to gather more data on how it performs. Enrollment highly encouraged.
But as we’ve demonstrated in a modeling study, injectable CAB-RPV doesn’t need to be 100% effective in a population with advanced HIV disease to save lives — even a virologic suppression rate of 45% would do the trick.
Did I win the bet? Or did Ken? I say we both save our nickels, and call it a tie.
February 20th, 2024
Variability in Consult Volume Is a Major Contributor to Trainee Stress — What’s the Solution?
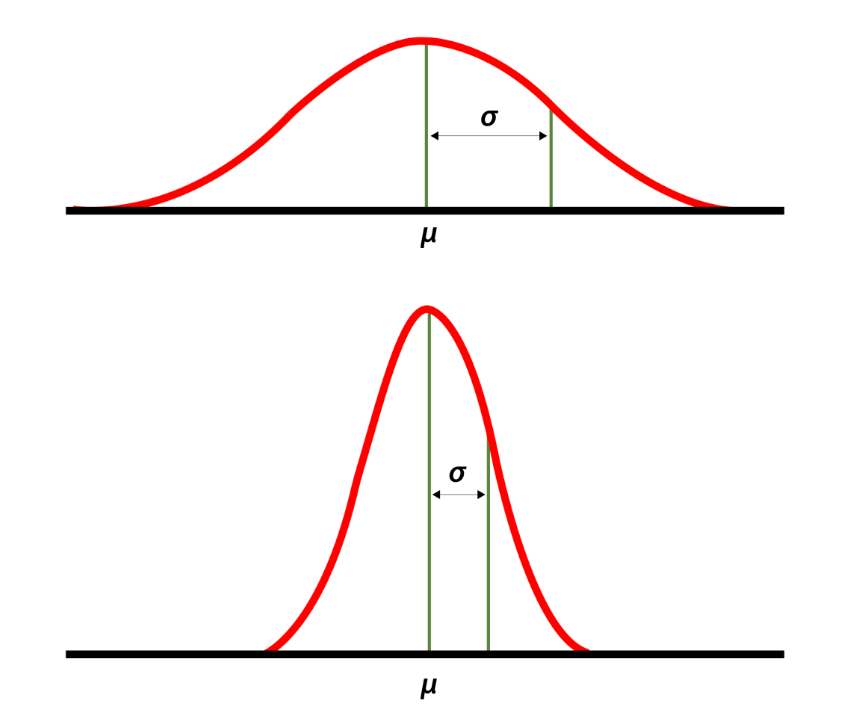
High and low standard deviations around the mean. Source: National Library of Medicine.
Back when he was program director of our ID fellowship, Dr. David Hooper would give the applicants a description of our program. One of the key parts was his estimating the workload — in particular, the number of new consults per day.
“We average three to four consults a day,” he said. “But there’s a high standard deviation around the mean.”
That last part he said humorously, with a smile and a shrug. It was a wonky joke, but everyone got it since it’s well known that consult volume is unpredictable — nothing different about our program compared to any other. But this variance is a critically important part of consultative medicine, one I’d argue is one of the key drivers of physician stress, especially for trainees.
What do I mean? Join me in this thought exercise. You’re an ID fellow with a weekend off, and it’s Sunday night. You’ll be picking up a new service on Monday — first day of a new rotation! — and will be responsible for learning the details of the cases on your team and catching up on weekend events.
Not only that, you’ll also be seeing the new consults that get called in that day. You know Monday will be busy, but how busy?
Let’s take two scenarios for the number of new consults — which would you choose if given the option?
- A day when you will get four new consults — no more, no less. Once you’ve done the fourth, you’re done with new consults for the day.
- A day where your consult volume that day is uncertain. You know that the average number of consults/day in your program is between three and four; however, light days (one or two consults) are balanced out with very busy days of five, six or, rarely, even seven new consults.
I suspect most of us would choose the first option, even though the “average” outcome of choice #2 is fewer consults.
This says a lot about psychology, risk perception, and our decision-making strategies. In studies of decision science, participants often choose the “sure thing” over a potentially more valuable but uncertain outcome — a phenomenon often referred to as “loss aversion.”
An important point is that the relationship between consult volume and stress does not increase linearly — it’s more like on a log scale, which means that going from five to six consults is much more difficult than going from three to four, even though both just add a single new case. And what this additionally means is that getting six consults is much more than twice as stressful as getting three.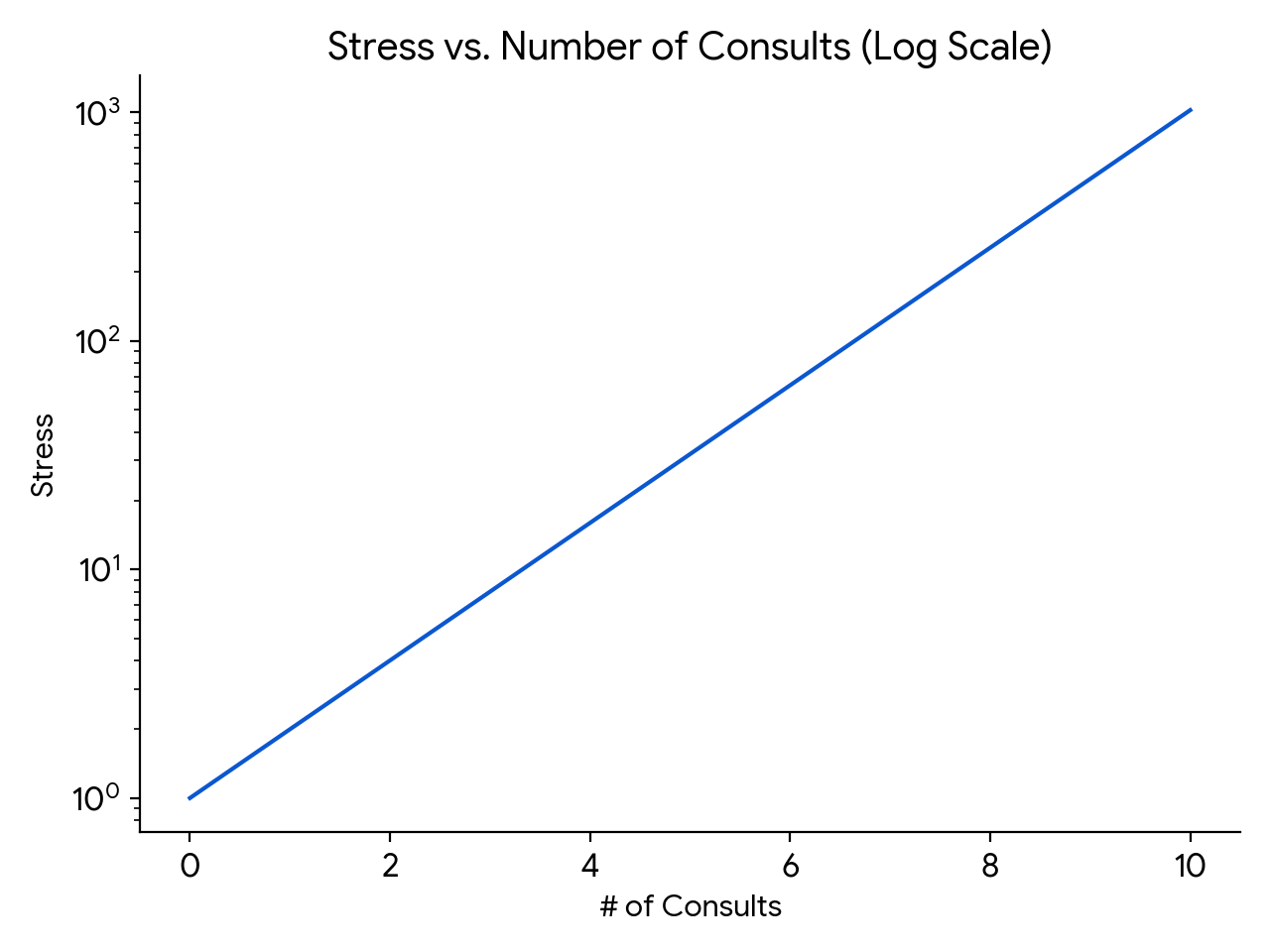
(How about those math skills. Impressive, eh?)
Finally, there is something inherently stressful about living through the amorphous blob of work potentially coming your way in choice #2. The day could start out relatively peacefully, with just a single consult, making you cautiously optimistic but still vulnerable. But then, an hour or so after lunch, the chief resident in orthopedics could page you and say they’ve just accepted in transfer two patients with infected prosthetic joints — both of whom will need your attention when they arrive (whenever that will be).
That hypothetical day still didn’t yield more consults than in choice #1, but the unpredictability of the way they came in made the day seem so much more tense.
(Note: This discussion must seem foreign to ID doctors in private practice, where consult volume directly links to personal revenue. But try to imagine yourself back in the days of your ID fellowship, however, and you’ll get what I mean!)
I thought of this challenge recently since we recently had quite the week when it comes to consult-volume variance. Afterwards, I sent a note commenting about this to Dr. Daniel Solomon, our fellowship’s current Associate Program director. His response:
I think the hardest thing about being on service (and in particular first-year fellowship when they are on the front lines holding the pager) is not the cumulative volume of work. It’s the unpredictability of each day. It is hard to make reliable plans with friends and family when the variance is so high.
I couldn’t agree more.
Solutions? One thing we proposed was to unload some of the simpler cases to an eConsult system, where inpatient medical and surgical teams received clinician-to-clinician advice from us after a discussion, record review, and our writing a brief note in the chart. The issue? As I wrote previously — no one has figured out how to pay for these things. Proposal rejected.
Some say that instituting a “cap” on new consults solves this variance-in-volume problem, and there’s definitely some truth to that. Such caps limit the burden of a high consult day on the ID fellows, much as a cap on admissions does the same for interns and residents. Plus, that uncertainty factor is greatly reduced.
This solution isn’t straightforward to implement, however. First, who sets the right number? ID training programs have a wide range of expected new cases per ID fellow per day. I’m very much aware that our daily average of three to four per day isn’t the same as other programs, some of which have considerably higher volume.
Also, should the cap be the same regardless of the number of patients you’re already following, or the complexity of the service? Should it be the same for general ID consults as it is for transplant and oncology services, which have an average complexity per case that’s much higher? And how do we account for variable team structures? Some regularly have rotating medical residents and/or students on board to help defray some of the work, while others rarely have these learners.
One other issue with a cap relates to the inherent value of clinical volume for volume’s sake. There’s a cliché in clinical medicine that goes, “The more you see, the more you see.” Since many ID programs (ours, for example) have only 1 year of intense inpatient clinical training, why not make the most of it, provided the volume isn’t too brutal? We all know that there’s no better way to learn about a clinical entity than to care for a patient who has it — the first-year ID fellow who sees CNS nocardiosis, or falciparum malaria during pregnancy, or disseminated histoplasmosis will never to forget those distinctive but relatively rare diseases, to choose just three that recently popped up on our inpatient service.
Although it doesn’t seem so at the time — an understatement — even doing a consult on “routine” cases brings value. Seeing many examples of Staph aureus bacteremia, or infected abdominal collections, or osteomyelitis under sacral pressure ulcers cumulatively helps develop an approach to these common entities, and to appreciate the wide variability in clinical presentation and management.
So far this discussion about the cap looks at it from the fellow perspective. It doesn’t address the fundamental cause, which is that the consulting services need our help caring for their patients, and it’s our mission to help them. That the volume of these requests is unpredictable isn’t their fault. Hence, once a trainee is capped, this work then must get done by someone else, right? Is it the on-service attending, who is still responsible for staffing the fellow-seen cases on this already busy day? Some other faculty member waiting in the wings, eager to do a late afternoon bunch of consults? Who has those faculty?
In summary, there are pros and cons to putting a cap on new consults for ID fellows. I’d be interested to hear — do you have a cap on new consults in your fellowship program? If so, what is it, and how did you decide on a number? Who’s responsible for doing the work over the cap? Share your thoughts in the comments section.
And enjoy this quite remarkable video, which somehow escaped my notice when it first appeared. Glad they remembered to press the record button!
January 25th, 2024
Printed Medical Textbooks — Going, Going, but Not Quite Gone
Take a look at the things behind my desk at work:

- cute photos of family and dog
- a bunch of sentimental objects, gifts from grateful patients or colleagues
- a smattering of miscellaneous plaques and clocks
- pictures of our current (awesome) first-year ID fellows and other stuff
- a bunch of books, several of them many inches thick
You may not be familiar with item #5 on this list — those hefty books — so allow me to remind you what they are. Those are medical textbooks. Because if you’re like me, there’s a decent chance you haven’t opened a medical textbook in months, or even years. It’s these that we’ll be chatting about today.
There was a time when consulting these heavy tomes fortified our approach to clinical care on a regular basis. Now, many of us barely ever open them; instead, we look online to recently published papers, comprehensive reviews, the latest treatment guidelines, or other sources to stay up-to-date.
(See what I linked there? Full disclosure, I’ve been a contributor to UpToDate since its very early days, as I’ve detailed previously.)
For evidence supporting this move toward online sources and away from print textbooks, take a look at the results of this poll:

Granted, those who respond to polls on Twixter already live much of their lives online, and hence least likely to turn to an actual printed book. That’s why I repeated it below — different audience, maybe a different response. But still, if you’re a doctor, nurse, pharmacist, or student, chances are excellent you own one or more textbooks, but they’re sitting somewhere in your office, their hundreds (or even thousands) of pages as fresh as the day they left the publisher.
Here are some representative comments I received related to this post:
I literally never use paper textbooks for anything and haven’t for about a decade.
I’m 35 and have a deep nostalgia for physical print books, so I have all my old textbooks (including Harrison’s), but I never use them.
Textbooks in print? Never. Last time over 15 years ago. I do read from the textbooks online.
Have these books in my office shelf for the sentimental value. I have used them twice in last few years.
Wrote a chapter in a textbook. Received free copy. Can’t find a med student around here who will take it off my hands.
Several of these textbooks might include outdated information, pointing to a critical problem with printed books highlighted by Dr. Michael Calderwood:
They look pretty on a shelf but are already outdated by the time that they arrive. Knowledge is changing quickly, and we need readily accessible, expert curated, living documents.
Even worse, how many of us replace the old versions? Guilty as charged — I checked the publication dates on some of the textbooks displayed on my shelf. One is from 2003! Another, 1997! No doubt there are some clinical pearls in these old books, but my advanced math skills calculate that they are decades outdated. Reader beware!
Or maybe your textbook serves another function:
In our fellows’ room, we have an old version of Mandell holding up a computer monitor (which occasionally displays the electronic pdf from the new version). That’s how we use it.
Importantly, there was a vocal contrasting opinion, no doubt representing the 10-28% from the poll. And it’s so interesting that it’s not just older doctors who have this favorable view of printed textbooks! Dr. Courtney Harris, a recent graduate from our ID fellowship program, weighed in with this comment:
I love them. I probably pull one out once a month for hard clinical questions or when I just need a deep overview of something I feel like I really don’t have a good grasp on. Plus I love physical books in general – having a few in my office just gives me joy.
This minority but opinionated group must help sustain the traditional textbook market. Some who continue to use print textbooks say that they can’t learn things as deeply from online sources — a reminder that there’s no right way to learn, just that people take different paths to their own education.
But going back to the numbers from the poll, I maintain that print medical textbook users represent a small (and I suspect shrinking) group. I wonder how long this fragile relationship will continue, especially because writing, producing, and publishing these books isn’t cheap — and neither is their price tag should you want to purchase one.
I confess that exploring this topic fills me with a tinge of nostalgia, one almost reaching melancholy. It has a similar emotional effect to hearing the accounts of other print media dropping in popularity and becoming decreasingly viable economically. Newspapers and magazines (in particular) have been decimated.
Augmenting the sadness is my longstanding participation in writing for some of these texts. For generations of doctors, an invitation to contribute to these pivotal books signaled a true honor. You felt a deep responsibility to research and know the topic thoroughly, to get it just right. After all, someone reading it could make treatment decisions based on your summary of the existing literature. Authorship in these well-known reference books acted as a good way for academic physicians to plant a flag that reads, “I belong.”
Today? A colleague of mine recently submitted a revised version of a chapter to one of these pivotal texts, one that I co-author with him. As it was being revised, I seriously wondered who would ever read it. I can’t imagine the numbers would come anywhere close to a comparable topic in UpToDate, or as covered in a treatment guidelines paper.
I wouldn’t give up on the printed stuff completely, though. Not only are there still people like Dr. Courtney Harris who still value the concrete, real thing you can hold in your hand, but perhaps there’s a burgeoning market: my 20-something-year-old daughter told me recently how much she loved subscribing to the print versions of two high-quality magazines.
They arrive in her mailbox each week — her actual mailbox, not the electronic one. Imagine that.
January 2nd, 2024
Reflections on Working in the Hospital During the Holidays

On-service ID team, December 25, 2023
For the zillionth year in a row, I spent the Christmas holiday working in the hospital. For me, it’s not much of a sacrifice — we don’t celebrate Christmas, and my kids are long out of school so the strict limits on when we can take vacation are a thing of the past. But it puts me in a notable minority, as around 90% of U.S. workers have the day off.
So what do we 10%-ers get in exchange for working? Here’s a secret — we get plenty. A brief rundown:
Camaraderie. This is one of the best things — the immediate recognition among those in the hospital that we’re in this together. Take a look at that picture of the faculty and fellows on call. Do those look like unhappy doctors? Importantly, a couple of the members of this quintet do celebrate Christmas, yet made the sacrifice to come in. To them, an especially big thank you!
Continuity. Sadly, illness doesn’t take a holiday and neither do hospitals — they’re a 24/7 business. The patients who must be in the hospital over Christmas are mostly quite sick, or socially disadvantaged, or both. They’re usually pretty upset about having to be there, quite understandably. So they very much appreciate that some of the doctors who were following them before the holidays know them during the holidays too.
Barter. Working Christmas allows us to trade this important holiday for others. For example, I haven’t worked Thanksgiving in years and almost always find a way to get coverage for Passover. Already I’ve blocked off Christmas 2024 as a time both my wife and I will be working — you’re welcome, happy to do it.
Bonus. Several years ago, our division adopted the policy of providing a small bonus to the on-service faculty for working certain important holidays. (Apologies to our residents and fellows who don’t get this. Maybe that will change now that they have adopted a union?) Note that for most salaried workers, it’s typically 1.5 or even 2 times the hourly rate. Believe me, it’s appreciated.

Golden Citizenship Award
Citizenship. Some of the crew on call over Christmas volunteer to do so nearly every year. As the person in charge of clinical scheduling, I hereby give them the Golden Citizenship Award. Mike, Eric, Amy, Anna — you know who you are — thank you! Place this award in a prominent place, it’s quite valuable.
Sustenance. The hospital president every year sends around an email wishing us happy holidays and announcing that lunch and dinner in the hospital cafeteria are free. This year’s entrees were pretty good, both the chicken and vegetarian options, though I wasn’t a big fan of the dessert. But if it’s sweets you’re after, no worries — hospitals are replete with goodies, homemade and otherwise, practically everywhere you go. This year, I arrived on one hospital unit to find gorgeous homemade chocolate chip cookies invitingly set out on a tray with a sign saying, “Take One”; another unit had a huge basket of assorted Lindt truffles. Noticing my rapt attention to the chocolates, a kind nurse insisted I not leave the floor until taking a few. Yum.
Appreciation. If you work over the holidays, you can pretty much guarantee that plenty of people will thank you. Patients, doctors, nurses. Hey, note that even I am thanking people (see “Camaraderie” and “Citizenship”, above).
Learning. Medicine is a lifetime of education! No reason this needs to stop over the holidays, right? Cryptosporidiosis, vascular graft infections, bacteremia as a complication of appendicitis, drug-induced vasculitis — summarized these topics and others in an on-line thread. Plus, one of the fellows shared an interesting paper on antibiotic-associated neutropenia. Much appreciated, Carlos!
Pace. Hospital census used to drop dramatically in the week before and after Christmas, leading to a delightfully slower pace in what is usually a very busy environment. While that effect is much less dramatic with the post-pandemic bed crunch, there are still fewer elective surgeries and admissions. So it’s still not quite as busy as during the rest of the year, a welcome break. And if you’re worried that I’m going to jinx this effect by even mentioning it, I refer you to a classic paper.
Diversity. Here’s a wonderful thing that made me smile on December 25, courtesy of Dr. Vamsi Aribindi:
Happy New Year, everyone!
December 22nd, 2023
A Holiday Season 2023 ID Link-o-Rama

Hey Image Creator, make a holiday greeting card in mid-century modern with dogs.
A bunch of ID (and a few non-ID) items of note as you prepare for the peak of the holiday season.
The President of IDSA has written an open letter to the American Board of Internal Medicine (ABIM) asking for changes to the recertification process. Current IDSA President Dr. Steven Schmitt proposes several modifications, all designed to reduce the burden and cost to the doctors. It importantly includes results from a member survey that shows a majority of ID docs believe the program “adds no clinical value, does not positively impact clinical practice and contributes to burnout.” A whopping 92% of early career ID doctors find the cost a burden. Note that it’s hard to find any survey that has responses so overwhelmingly negative about an ostensibly educational program.
Here’s part two of my commentary on this ABIM issue. In this update, the current president of ABIM gets mad, says something he later regrets (maybe), apologizes (accepted, thank you!), but sticks to his guns anyway. After reading this, my ID colleague/pal John Badley kindly sent me an email stating, “Paul, you and your blog are not despicable.” Thanks for the reassurance, John! And let me be clear once again — I do believe that it’s essential physicians continue to learn, and to keep their clinical practice updated, but I’m increasingly aware that the recertification process as currently designed has major limitations.
Patients who had recently used PrEP and acquired HIV were 7 times more likely to have baseline resistance at HIV diagnosis than those who never used it. The most likely explanation for this excess resistance is that PrEP was stopped, and then re-started after HIV was acquired. The most common mutation detected was M184V (lamivudine and emtricitabine resistance), so DTG and BIC-based three-drug therapy still should be active. Rather than use these data to discourage use of PrEP, we should leverage it to encourage better adherence and more regular follow-up.
Two investigational antivirals hastened time to recovery in people with COVID-19 compared to placebo. VV116 is a remdesivir oral analogue, and leritrelvir is a SARS-CoV-2 protease inhibitor given without ritonavir. Neither study reported significant safety concerns, and both would have fewer drug interactions than nirmatrelvir/r (Paxlovid), especially VV116. While the authors didn’t comment on rebound, recall that this issue does not happen with IV remdesivir. These two drugs (VV116 in particular) along with ensitrelvir all appear to have major advantages over our existing options for outpatients. As cases rise again this December (as they have every December since 2020), I eagerly — and confess impatiently — await better alternatives!
For children presenting to emergency departments with acute gastroenteritis, molecular testing led to a significant increase in identification of a potential pathogen and decreased the need for follow-up visits. The diagnostic yield increased from 3% in the pre-intervention period compared to a whopping 74% with molecular testing. Molecular testing was associated with a 21% reduction in the odds of any return visit, a clinical benefit strongly endorsed by my pediatrician wife (fewer “he had another loose poop” calls). These results remind me of one of the early studies of enterovirus PCR for meningitis, showing that even when you don’t have a treatment, sometimes just knowing the diagnosis can have benefits.
Treatment of men with UTIs and fever is one of the exceptions to the “shorter is better” rule of antibiotic courses. More relapses and treatment failures occurred after the 7 vs. 14 day course; strongly suspect prostate infection is the nidus for recurrence. I’d add high-risk vertebral osteomyelitis to this short list of conditions that may require longer treatment, based on both a prior observational study and anecdotal experience.
A microbiology laboratory tested 85 clinical isolates of beta streptococci for susceptibility to trimethoprim-sulfamethoxazole, clindamycin, and doxycycline. The surprise (to some) winner? Trim-sulfa (100%), followed by clindamycin (85%), with every ID doc’s favorite doxycycline bringing up the rear, with only 57% susceptible. Some would argue that this in vitro activity of trim-sulfa vs. strep has long been proven in clinical studies of skin and soft tissue infections (such as one in kids and one in kids and adults), but that hasn’t stopped clinicians from frequently choosing double therapy with beta lactams plus trim sulfa for outpatient treatment of these infections. Guidelines, too, steer people away from trim sulfa for strep. Trim sulfa’s bad reputation vs. strep stems from an artifact introduced in a now outdated method of resistance testing, which had high thymidine content of the test media inhibiting the activity of the drug.
Weight gain after switching an NNRTI-based regimen to tenofovir DF/lamivudine/dolutegravir occurred only in those whose pre-switch NNRTI was efavirenz — not nevirapine. As previously observed, the women gained more weight than men. Also previously noted is that efavirenz has a weight-suppressive effect, which is not observed in other drugs in the NNRTI class — rilpivirine and doravirine also do not suppress weight. The mechanism by which efavirenz has this effect remains unclear, but I’m betting that this is an off-target toxicity. One confounder in this study is that most of the participants on NVP were on AZT/3TC, most on EFV were on TDF/3TC, and these NRTIs also influence weight.
In a placebo-controlled, randomized clinical trial, vitamin D did not lower the risk of upper respiratory tract infection in older adults. A large study, involving 16,000 participants, this is another negative vitamin D supplementation randomized trial we can add to a large pile. Oddly, in a prespecified analysis, the researchers observed a significantly lower occurrence of URI in the vitamin D group, compared to placebo, during summer only — exactly the opposite of what I’d expect. A protective effect also was seen in Black participants.
A synbiotic preparation called SIM01 improved certain symptoms of long COVID significantly more than placebo. These symptoms included fatigue, “general unwellness,” memory loss, gastrointestinal upset, and difficulty in concentration. There was a corresponding alteration in the treatment group’s gut microbiome. Confess I didn’t know what constituted “synbiotics” before this study — it means “a mixture of probiotics and prebiotics that beneficially affects the host by improving the survival and activity of beneficial microorganisms in the gut.” And in case you’re counting, this is the third COVID-19-related randomized clinical trial coming from China I’ve chosen to highlight.
A graphic designer provided a detailed account of her devastating symptoms from long COVID. This is a long, quite heartbreaking piece, beautifully depicted, painful to read. Clinicians will appreciate that for some patients, the meticulous record keeping provides some solace, the sheer volume of it starting in 2020 no doubt correlating with the severity of her symptoms. She writes, “I thought that if I collected enough data, I would eventually figure out what was going wrong. But no matter how much data I collected or how many correlations I tried to draw, answers eluded me. Still, I couldn’t stop tracking. My spreadsheet was the only thing I could control in a life I no longer recognized.”
A detailed pharmacokinetics study evaluated bictegravir/FTC/TAF in 29 virologically suppressed pregnant women with HIV. The main findings were that while concentrations of all three drugs during the second and third trimester were lower than postpartum levels (roughly 40-80% of postpartum levels, biggest reduction for bictegravir), the mean bictegravir exposures were still more than 6.5-fold greater than the protein-adjusted 95% effective concentration. No virologic failure or infant transmissions occurred. This regimen — the most commonly used in the U.S. right now — is still listed as having “insufficient data” in pregnancy guidelines. I wonder if this small study will change that category.
Latent infection with Toxoplasma gondii was associated with increased mortality. Note that I wrote “associated” and not “causes”, as this large observational study found marked demographic differences between those who were seropositive and seronegative. One also wonders why the tests were sent to begin with — certainly it’s not a commonly ordered test, done only in very specific clinical circumstances. Nonetheless, it’s worth remembering that even latent infections can have consequences, as is increasingly evident with studies on CMV.
A Mostly Non-ID Section:
The story behind the “booming business” of cutting under a baby’s tongue to improve breastfeeding. Although well intentioned, this procedure has minimal evidence to support its now growing use despite being widely recommended by certain lactation specialists. It also takes advantage of parents during a time of great emotional vulnerability when there is already enormous pressure to breastfeed. Here’s what we do know: it’s very profitable for the doctors and dentists doing the procedure, and it can have serious complications. I’ve been listening to wise pediatricians — especially one very close to me! — express concerns about this procedure for years, so it’s gratifying to see this exposed.
A survey of nearly 19,000 physicians found that nearly a third had a moderate or high “intention to leave” practice within 2 years. With the caveat that any survey may select only for those who are unhappy and want to express their discontent, the most striking figure from the paper places medical specialties in 4 different quadrants — along the vertical axis professional fulfillment, the horizontal proportion with burnout. Trust me, we don’t want to be in the bottom right (low professional fulfillment, high burnout). I was pleased to see that ID just escaped, sitting in the upper right — scoring higher than average on the burnout side of things (bad), but also higher on professional fulfillment (good)
A general internist wrote about the patients who fired him. In this excellent account of a difficult subject — and the valuable things he learned through the process — he tells the story of four such patients who left his practice. He concludes by writing: “To keep this reflection under 2000 words, I leave out the stories of two other patients. I am also not sure I could have tolerated writing more.” We need more of these stories in clinical medicine to balance out the “I came in and saved the day” anecdotes — which not surprisingly are much more common!
Hey, happy holidays everyone! Let’s wrap up the year with these American-born dancers living in Galway performing to a song written and performed by a singer-guitarist from Puerto Rico, who quite amazingly was born blind and started playing the guitar at age nine.

