An ongoing dialogue on HIV/AIDS, infectious diseases,
February 13th, 2017
How to Make Preventing Heart Disease in HIV Fun and Exciting: The REPRIEVE Trial
The people researching cardiovascular disease in HIV have quite the challenge.
Because when you think about it for a second, we HIV treaters are a pretty spoiled bunch when it comes to therapeutic success.
We saw the transformation of a terrifying, incurable, and rapidly progressive disease (AIDS) into something that can be managed for decades — usually with just a pill or two each day.
Or, if you prefer a quantitative description, here’s the slide Tony Fauci uses when he cites my friend Rochelle Walensky’s 2006 landmark paper in his talks:
Remember, these impressive numbers were estimated before we had the great advances in antiretroviral therapy in the late 2000s. The benefits are undoubtedly even greater over the last decade, since virtually everyone who takes HIV medications regularly now is virologically suppressed.
Pretty much any medical intervention for a non-HIV problem might seem a bit ho-hum compared to the miracle of antiretroviral therapy, meaning the people researching non-HIV related problems have their work cut out for them to get our attention. This is despite the fact that these non-HIV problems (especially the problems of aging) are more important than ever.
So when Steve Grinspoon contacted me recently about mentioning the REPRIEVE study on this site in honor of “Heart Month”, I readily agreed. Steve is leading this large NIH-funded trial, which asks this important question:
If people with HIV are intrinsically greater risk of heart disease, would they benefit from taking a statin drug even if we would not ordinarily prescribe it based on standard risk assessments?
REPRIEVE is actively enrolling at clinics and hospitals around the world, including at our site here in Boston.
As demonstrated in multiple studies (and reviewed here), people with HIV do appear to be at higher risk for heart disease even when their virus is well controlled with ART. “Lifestyle” issues (especially smoking), current and past immunosuppression, duration of uncontrolled viral replication, cardiotoxic drugs, and immune activation and inflammation all likely play a role. Related to the issue of inflammation, Steve’s research group just published a paper demonstrating that abnormal arterial inflammation — a risk factor for coronary disease — continues despite effective ART, at least in the short term.
So why not just recommend a statin for all people with HIV above a certain age? While it’s theoretically possible that statins will improve this abnormal inflammatory state, we don’t know that statins will provide a clinical benefit. And perhaps not all people with HIV are at higher CV risk — the most recent look at MI incidence in the Kaiser HIV cohort did not find an excess risk in a group of very healthy (high CD4, modern ART regimens, low rates of smoking) HIV patients compared to HIV negative controls.
Which brings us back to REPRIEVE — statins might be beneficial, they might not, and there’s enough equipoise that it’s appropriate to conduct a real randomized trial.
Eligible patients must have stable HIV, be older than 40, and not have a high risk for heart attacks or stroke (otherwise they should be on a statin). If you want to check, you can enter patient information into the American College of Cardiology/American Heart Association heart risk calculator. Those with a 10-year risk of heart disease or stroke < 15% may be eligible, depending on LDL cholesterol levels.
Participants are then randomized to pitavastatin (chosen because it doesn’t interact with any HIV drugs) or placebo. The primary endpoint is time to atherosclerosis-related clinical event.
I think it’s an excellent study, not just because I’ve known Steve for ages and he’s been a leading figure researching metabolic complications of HIV disease longer than just about anyone. A clinical trial that evaluates a critical non-HIV related complication makes abundant sense in a population where infectious complications are increasingly rare. The results will have great importance to patients, in particular the increasing proportion over the age of 50 (which now exceeds 50% in many urban areas).
Plus, if I mention REPRIEVE here, maybe I’ll get him to read this blog — which he obviously had never done before he called me. “I’ve been really busy,” he said.
Too busy to read Fun With Old Medical Images? To weigh in on whether ID doctors are the worst dressed specialists? To talk about doctors named Morgan? Or if we should wear white coats?
“Really busy”? — not sure I’m buying that excuse!
February 5th, 2017
Case Report of PrEP Failure: What Can We Learn From It?
The New England Journal of Medicine has published the first well-documented case of HIV pre-exposure prophylaxis (PrEP) failure despite good medication adherence.
We heard lots of this information at CROI last year, and again I’m impressed at the extraordinary degree of virologic investigation done on a case from clinical practice.
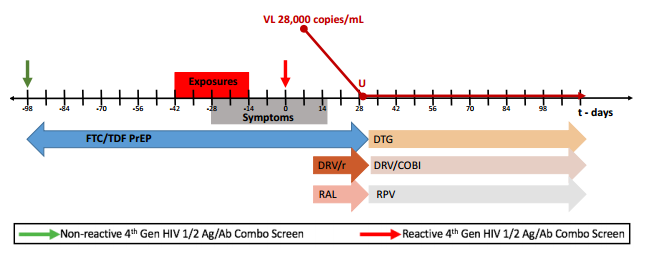
To refresh your memory, here are the critical details from the published case report and the supplementary appendix:
- A 43-year-old man began PrEP with TDF/FTC in April 2013, and had multiple negative 4th generation HIV screening tests over the next 21 months.
- Pharmacy refill records indicated excellent adherence. (Side note to young clinicians: Refill frequency is an amazingly powerful tool to monitor adherence.)
- Prior to diagnosis, he had multiple sexual exposures, including receptive anal intercourse without the use of condoms.
- A couple of weeks before his first positive HIV screening test (antigen positive, antibody negative — Day 0), he developed abdominal pain which waxed and waned over the next 3 weeks. A CT scan demonstrated thickening of the sigmoid colon, ascending colon and rectum. Endoscopy revealed erythematous patches of the sigmoid colon.
- Adherence was confirmed by analysis of a plasma sample obtained on Day 0 revealing tenofovir concentrations consistent with recently taking the drug.
- HIV RNA peaked at 28,000, and became undetectable with the addition of boosted-darunavir and raltegravir.
- His viral isolate had extensive multi-class resistance, including to FTC (M184V), TDF (multiple thymidine-associated mutations), NNRTIs (Y181C), and first-generation integrase inhibitors (92Q).
- He’s now virologically suppressed on DRV/c, RPV, and DTG.
The supplementary appendix has an excellent figure of the timeline, which I’ve pasted below:
So what can we learn from this single case?
- PrEP is very effective, but it’s not 100% protective. I don’t think clinicians are claiming 100% effectiveness, but PrEP-takers may be hearing this from various “experts”, or may be misunderstanding the data.
- Transmitted drug resistance will weaken PrEP. Although all of the mutations in the case are to drugs not in the TDF/FTC combination — except M184V — the multiple TAMs could have weakened TDF through cross resistance. Furthermore, FTC could have been transmitted, or alternatively selected by period of viral replication after viremia developed. The source patient’s virus was not available for sequencing, unfortunately.
- The symptoms of acute HIV infection are highly variable. The supplement to the case reports states, “The patient did not have classic symptoms of acute HIV seroconversion”, which is true — but he did have GI symptoms, which are quite common in acute HIV. We need to remember this variability when following patients on PrEP.
- The monitoring strategy recommended by the CDC in the guidelines should be followed. Although it might seem like overkill to monitor for HIV and other STIs every 3 months while receiving PrEP, remember that only those at highest risk for getting HIV should be receiving PrEP to begin with. Clinicians may choose to recommend more frequent monitoring if clinically warranted.
- Integrase resistance is rare in clinical practice, and transmitted integrase resistance is even less common. While some might interpret this case report to indicate a need for baseline integrase testing for newly diagnosed individuals, the detection of transmitted integrase resistance in most studies of newly diagnosed patients is less than 1%, and often 0%. Notably, the transmitted virus was still sensitive to dolutegravir (I’d interpret the genotype as fully susceptible).
Ok, that’s what I learned. Provides a cautionary note to those both prescribing and receiving PrEP, one further reinforced by this additional case report of PrEP failure presented at a recent conference.
As for tonight’s game, below is what I’m going to watch, and here’s why!
[youtube https://www.youtube.com/watch?v=15SdPnof0FY&w=560&h=315]
January 29th, 2017
In A Weekend of Paranoia and Anger and Fear, Some Release From the Other Side of the Globe
 This was not a happy or comfortable weekend for ID doctors, given our predilection for inclusiveness, non-judgmental care, global health, and that “safety net” idea that seems to us such an intrinsic part of being a good doctor.
This was not a happy or comfortable weekend for ID doctors, given our predilection for inclusiveness, non-judgmental care, global health, and that “safety net” idea that seems to us such an intrinsic part of being a good doctor.
Exclusion of foreigners? Why, we ask, would you do that? People from other countries are our friends, our colleagues, and our patients. Many of our favorite patients, I might add, since they might bring with them exotic, challenging, and treatable problems that are right up our alley.
Furthermore, to overstate the obvious, most of us living here in the USA are not that far removed from immigration ourselves.
Fidencio Saldana — he’s a cardiologist, the Dean of Students at Harvard Medical School, and the child of Mexican immigrants — gave medical Grand Rounds at our hospital recently.
He recounted what it was like being a medical student here, and how out of place he sometimes felt. He then movingly described how was made to feel welcome by hearing from one of our senior doctors, Marshall Wolf:
Hey relax. My parents were immigrants too. You belong here.
That was two weeks ago, a brief aside made at a lecture, yet I have thought of it frequently since, especially this weekend.
And this morning, glum and discouraged by recent events, I needed some distraction.
So I turned on the television at 6AM and watched two of the most extraordinary athletes in the world, Roger Federer of Switzerland and Rafael Nadal of Spain, play the fifth and deciding set of tennis at the Australian Open in Melbourne, Australia.
The crowd was cheering, and hanging on every point, and no doubt millions of others all over the world were doing what I was doing — watching and marveling and feeling a sense of global togetherness for this extraordinary event.
And here’s the best part. After Federer won the match, a grueling 5-set affair that lasted over 3 hours, he graciously said:
Tennis is a tough sport, there are no draws. But if there was I’d be happy to share it with Rafa tonight.
Share. No gloating. No petty rivalries. No cheating. No distortion of facts. These are the best players in the world, competing at the highest level for hours, and they can still get along like friends even in the most intense setting imaginable.
You got that?
https://youtu.be/upLv0xBTai8?t=125
January 22nd, 2017
Fun With Old Medical Images!
Welcome to Fun with Old Medical Images!
Here’s how it works: You’ll see a series of images — old, strange, and perplexing — and each will have a caption that I have created for you at no extra cost.
Accustomed to high-quality and clinically relevant information from your NEJM Journal Watch contributors, you will laugh happily at the contrast between that solid content and this silly exercise, forgetting for a moment the complicated and sometimes contentious world we live in.
See, this will be therapeutic.
Off we go. Here’s the first one:
“Perkins wondered whether his doctors might consider an alternative treatment for his migraines, as the heated box on his knee appeared to be ineffective.”
Poor Perkins! I’m sure he’s also wondering why his doctors didn’t give him a pillow.
That was fun. Ready for #2?
“Drug development of the novel sleep aid was halted when many patients reported lethargy, fatigue, and vivid dreams of milk, eggs, and lamb chops.”
So many promising drugs fail during clinical trials due to side effects! At least dreaming of lamb chops is safer than QT prolongation.
Now, onto #3:
Such stillness in that room! And who’s the uniformed man in the upper right? Is he a scribe?
We’re on a roll! Here’s #4:
That’s one smart girl — she reads the literature! And she knows for a fact that Andrew Wakefield (not in this picture) is a fraud.
The laughs keep coming, so let’s go on to #5:
Here the key question — did medical insurance in mid-19th century France cover the cost of Uber for housecalls? I did a quick search — fascinating result.
Should we finish with a neat half-dozen? Why not, here’s #6:
“Patients were thrilled with the new device, which both measured intraocular pressure and gave a quick hair blowout.”
Nothing but fun at this ophthalmologist’s office! Eye appointments always last too long, so why not get checked for glaucoma and get a bit more stylish at the same time?
Thank you for taking this tour of Old Medical Images, I hope you enjoyed it as much as I did.
Next time, we’ll go back to our usual programming.
January 16th, 2017
Two Case Reports Worth Reading, and Enlisting Pro-Vaccine Support
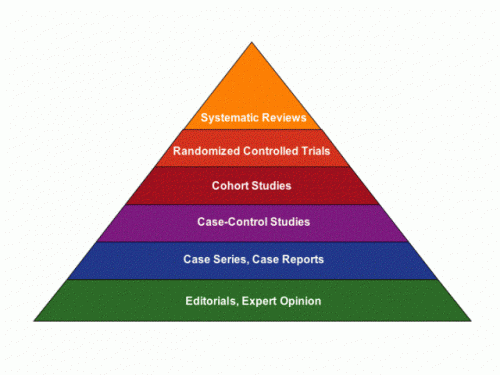 Case reports are pretty low down on the “levels of evidence” pyramid. This low status notwithstanding, when they are well done they can illustrate important clinical lessons, including these two:
Case reports are pretty low down on the “levels of evidence” pyramid. This low status notwithstanding, when they are well done they can illustrate important clinical lessons, including these two:
- A Las Vegas woman died after infection with a pan-resistant strain of Klebsiella. While CDC receives many isolates of carbapenem-resistant enterobacteriaceae (CRE), 80% are susceptible to at least one aminoglycoside and 90% to tigecycline — not this one, which retained susceptibility only to fosfomycin (a drug not available in this country for systemic use). One critical detail in this case was her multiple recent hospitalizations in India related to a right femur fracture and osteomyelitis of the right femur and hip. As with this case of metronidazole-resistant Bacteroides, the travel appears to be the critical exposure history. Question for those who practice in a Travel Clinic — do you now counsel patients about their risks of acquiring highly resistant organisms during travel to certain countries? Seems this risk is particularly high with travel to Southern Asia — probably higher than many of the more exotic conditions that get more attention.
- A man experienced a delayed diagnosis of TB meningitis due to a false-positive multiplex PCR. A 75-year-old man presented with mental status changes, and the BioFire FilmArray on the CSF was positive for HSV-1. Herpes encephalitis, case closed, right? However, the ultimate diagnosis was tuberculous meningitis, and the HSV-1 was not confirmed by additional PCR testing using another FDA-approved assay. Of course in hindsight, it seems clear that this wasn’t clinically consistent with HSV-1 encephalitis, FilmArray result notwithstanding — onset of confusion and speech difficulties was gradual, not acute; an MRI showed no parenchymal abnormalities, unusual in someone with HSV-1 encephalitis of this duration and severity; there was a neutrophilic pleocytosis and markedly elevated CSF protein; he developed hydrocephalus. Individually, several of these could rarely be seen with HSV-1 encephalitis, but together along with his clinical worsening they appropriately raised the concern that the diagnosis was incorrect. Of course hindsight is 20-20, and the most important message in this case is not to succumb to “premature closure” if the clinical picture does not fit the lab test result — an especially important lesson with new testing modalities.
Now, to enlist your support for vaccines, for which there is the highest level of evidence for efficacy and safety. At the kind invitation of Carey Goldberg, the skillful editor of WBUR’s CommonHealth blog, I wrote a piece about the possible appointment of Robert F. Kennedy Jr. by Trump to lead a commission on vaccine safety and scientific integrity.
Kennedy, you may be aware, has a lengthy history of pushing anti-vaccine propaganda.
To you, loyal readers of an ID/HIV blog, I am going to state with 99.9% confidence that you will agree with my views — but please read the piece anyway. This debate about the risks and benefits of childhood immunizations is still (amazingly), a thing — just read the comments if you can stand it — and we need all the support we can get.
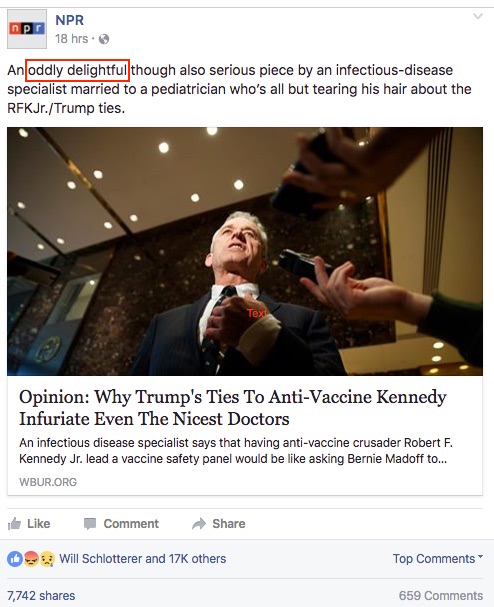
But I have a query. Here’s the NPR Facebook page on the piece, with one section highlighted for discussion:
Not sure exactly what they meant by “oddly delightful”, but I’m pretty sure it’s better than the converse — “delightfully odd.”
Happy MLK Day.
[youtube https://www.youtube.com/watch?v=cuAl5cMTJ7A&w=560&h=315]
January 8th, 2017
Poll: Should Medicine and Family Practice Residency Programs Have a Dedicated HIV Track?
A few medicine and family practice residency programs around the country have a dedicated track that focuses on HIV care. Though the programs naturally differ somewhat in structure — here are two examples from University of Washington and Yale — they generally involve placing the resident into an HIV clinic for their longitudinal outpatient experience.
We don’t have such a program here, though I’ve been asked about it several times over the years. I can certainly think of advantages and disadvantages to this specialized track.
And since we’re in the midst of residency interview season — plenty of young, bright people wandering around the hospital in dark suits they might not wear again for a couple of years — it seems a good time to consider the issue.
On the plus side for the HIV track:
- Residents with a stated interest in HIV care can get a head start on their career choice.
- There’s a projected shortage of HIV clinicians, and this training will help provide a capable and interested group of young doctors in the field. Residents can skip specialty training in ID and transition right to primary care with a panel of HIV patients.
- Under current training standards, program directors report a high proportion of internal medicine residency graduates are not adequately trained to provide primary HIV care.
- People with HIV are more likely to be poor, from minority or other traditionally marginalized communities (gay men or people with addiction), and having more clinicians sensitive to their needs certainly is a plus.
On the minus side:
- A focused HIV track arguably limits both the breadth of patient experiences and the ambulatory clinical challenges for the resident. Shouldn’t residents get as broad an education as possible at this early stage of their training? This is especially important if they change their minds and choose to do something else.
- If there’s going to be an HIV track, why aren’t there specialty tracks for other diseases? How about more common conditions, such as the primary care needs of cancer survivors, or people with mental illness, or diabetes? Or to choose a couple of problems with comparable numbers in the USA to HIV — how about adults with congenital heart disease, or those with lupus?
- Since so many patients with HIV today are completely stable from the HIV perspective, a dedicated HIV track isn’t necessary. The focus of residency should be learning how to manage problems of aging (hypertension, diabetes, COPD, cancer screening) since these are the most important issues for many HIV patients anyway. Data are emerging that a primary care/specialty collaboration works well — here’s a good recent paper evaluating this issue.
- Doctors who want to manage the most complex HIV issues — multi-class resistance, knotty metabolic abnormalities, opportunistic infections, challenging drug interactions — should do additional subspecialty training Infectious Diseases. In most clinical settings, these situations would prompt a specialty referral regardless of how a resident was trained.
I’m not going to pretend to have the answer to this one. That’s why there’s a poll, and the comments section!
December 25th, 2016
Ebola Vaccine, a New Use for Listerine, USPSTF on HSV, Nether Grooming, and More: A Christmas and Hanukkah Overlap ID Link-o-Rama
A few notable ID stories out there for this remarkable convergence in our Judeo-Christian holiday calendar:
- Experimental Ebola vaccine “100%” effective. Impressive scientific progress on prevention of this terrifying disease, with even better strategies expected soon.
- 10 days of antibiotics is better than 5 for childhood (age < 2) otitis media. For the record, my “inside source” on this question is not surprised one bit at the result. We’re seeing the opposite trend in sinusitis and pneumonia treatments of adults, toward shorter antibiotic courses.
- The USPSTF recommends against routine serologic screening for genital herpes. The serologic test is lousy, with lots of false positives — from the summary: “The positive predictive value may be as low as 75% for the biokit test and as low as 50% for HerpeSelect.” As I’ve noted before, the recommendation against general screening probably won’t apply to patients who come in requesting to be tested, where you can explain the pros and cons of the strategy.
- The American College of Cardiology/American Heart Association risk calculator discriminates cardiovascular risk “adequately” in people with HIV. Adding HIV-specific factors (HIV viral load, CD4 lymphocyte count, antiretroviral therapy, and protease inhibitor use) to the calculator did not improve the performance. A take away point is that at the lower estimates, these should be considered the minimum risk.
- Listerine is active against pharyngeal gonorrhea. At last, the answer

to the challenges of the post-antibiotic era! And time to bring back this classic ad campaign (see poster), with slight modification: “He’s not going to call — but the Department of Public Health will!” - Two-drug HIV therapy with dolutegravir and rilpivirine is non-inferior to continued triple therapy. Full results of these studies — called SWORD — are to be presented at a scientific conference, presumably at CROI in February 2017.
- IAS-USA released an update of the HIV drug resistance mutations. Even today, when emergent resistance to antiretrovirals is less common, this is a critical and authoritative resource for all HIV providers.
- The investigational HCV treatment glecaprevir/pibrentasvir has been submitted to the FDA. This protease inhibitor/NS5A inhibitor combination pill is a pan-genotypic approach that will be 8 weeks for treatment-naïve, 12 weeks for treatment-experienced or anyone with compensated cirrhosis. Clinical trials have included a wide range of patients, including those with HIV, advanced renal disease (including ESRD), and people who have failed current HCV therapy — the last of these granting a Breakthrough Therapy Designation, meaning likely fast approval.
- Think that because antibiotics in food is bad PR, there has been a significant decline in the food industry’s use? Think again — according to the latest FDA reports, sale of antibiotics to farms increased by 1% in 2015 compared with the year before.
- Topical azithromycin does not prevent Lyme disease. Oh well. On the plus side, there is at least some active research on a new Lyme vaccine.
- Phage therapy seems to have helped cure this case of multi-drug resistant Pseudomonas. It’s just a case report, and not (yet) in a peer-reviewed publication, but it’s quite the story nonetheless — and has prompted numerous non-ID doctors to ask me about it, especially in the ICUs. Fascinating list of potential non-antibiotics approaches to drug-resistant bacteria here.
- Results of anaerobic cultures rarely influence clinical management. In general, most of the patients with positive anaerobic cultures were already receiving therapy directed against the isolated bacteria, or there was no acknowledgement of the result at all. The authors call for anaerobic cultures to be ordered more selectively, which makes sense.
- Pubic hair grooming is associated with increased risk of certain sexually transmitted infections. In a study like this, the possibility of “confounding by indication” is astoundingly high (to be a stats nerd for a moment) — meaning that those who were extensively grooming may have been extensively doing other things, too! It remains a provocative finding nonetheless.
- If you’re cared for by a female physician, you’re more likely to survive. Maybe because so many of them choose ID and pediatrics!
Finally, it’s been a tough year for giants in music, especially if you’re a fan of a certain age. We lost Prince, David Bowie, Leonard Cohen, Leon Russell, Keith Emerson, Sir George Martin … and today, George Michael.
Here’s a somewhat less-famous song of his (certainly less flashy — here if you want that), with a nice late-Beatles/John Lennon quality to it, and lyrics quite appropriate to the times. Enjoy.
[youtube https://www.youtube.com/watch?v=goroyZbVdlo]
December 18th, 2016
Holiday Distractions, Dirty Pets, and Time to Vote for Your Favorite Cartoon Caption
 Many people find it tough to concentrate at work this time of year. So much to juggle:
Many people find it tough to concentrate at work this time of year. So much to juggle:
- Holiday parties.
- Shopping.
- Travel plans — specifically, should we go with DEET or picaridin?
- Lots of high-carb, high-calorie foods stealing blood from our brains. Maybe too much eggnog or punch doing the same thing.
- Kids on vacation, with their demands for sustenance, adult supervision, and entertainment.
- Many critical work support people already away, with out-of-office messages implying that they’re not reading their email (but you know that they are).
Even our pets are distracted. After a crazy weekend of New England weather, starting with bitter cold, then a day of wet snow, and now a Spring-like thaw, Boston dogs and cats who ventured outside today are in bad need of bath.
(And yes, that was just an excuse to show off that magnificent picture of Louie. Click on it for full effect, I dare you.)
Plus, you have the distraction of our latest ID Cartoon Caption Contest, which generated so many outstanding entries that our powerful advanced humor algorithm was unable to limit the finalists to just three candidates this time. Even after we tweaked the settings, the powerful supercomputer churning through the entries kept overheating. We almost had to cancel today’s post and substitute a lengthy rumination on whether you should say Mycobacterium avium complex (MAC) or Mycobacterium avium-intracellulare (MAI).
(Short answer: the former.)
So here’s the cartoon, plus the top four choices. Vote now before 2016 is no more!
(Drawing by Anne Sax.)
December 11th, 2016
You Want Guidelines? We Got Guidelines!
About a million years ago — in other words, probably sometime during my ID fellowship — I asked transplant ID guru Bob Rubin how various ID guidelines came together, including one on antifungal therapy he had just led.
“You lock a bunch of experts in a hotel conference room,” he said. “Provide them plenty of food and coffee. Then you hide the key until they come up with something, especially if there’s a deadline .”
Having participated on a couple of guidelines committees, I can attest that the process has changed just a bit since Bob provided me that memorable story. Conference calls, web-based presentations, and numerous shared documents with extensive “track changes” peppered throughout now dominate the process, along with frequent reminders about grading both the strength of the recommendations and the quality of evidence.
And, since we’re ID doctors, we of course try to outdo each other in obsessive attention to detail — emphasis on obsessive. While everyone makes a big noise about editing and shortening the final document, if that means eliminating one key reference, tell that to its champion — especially if they authored or co-authored the paper. It’s going in!
On the topic of guidelines, you might have noticed that the Infectious Diseases Society of America (IDSA) has been on quite a roll over the past year. They’ve been churning out new guidelines at a blistering pace, often in collaboration with other medical societies. So here’s a look back on IDSA’s hard work over the past 12 months, each one highlighted with at least one notable and/or interesting recommendation, and also (just for bragging rights) the number of references (all thoroughly read, no doubt):
- Candidiasis. Last updated in 2009, these revised guidelines cover diagnosis, prevention, and treatment. A selected key recommendation: An echinocandin is recommended as initial therapy, with transition to fluconazole if the isolate is susceptible. Number of references: 560 — impressive!
- Antibiotic Stewardship. The first of its kind! A selected key recommendation: Rapid viral testing for respiratory pathogens is recommended to reduce the use of inappropriate antibiotics. Number of references: 225 — you have to think this will grow in the next iteration.
- Aspergillosis. Prevention, diagnosis, and treatment of this difficult-to-treat opportunistic infection. A selected key recommendation: Serum and bronchoalveolar lavage galactomannan can accurately diagnose invasive aspergillosis in high risk patients; the panel disagreed about using PCR. Number of references: 655! Wow, that might be hard to beat.
- Hospital-acquired and ventilator-associated pneumonia. Retires the term “health-care associated pneumonia”, often abbreviated (and spoken) as “H-CAP” — will be hard for many to break that habit. A selected key recommendation: Duration of therapy should be 7-days (follows the rules!), not 8-15. Number of references: 364. Middle of the pack.
- Coccidioidomycosis. What a mouthful that fungal disease is, which must be why most people just say “cocci.” A selected key recommendation (actually two this time): No antifungal treatment for an asymptomatic pulmonary nodule (not even a bit of fluconazole?), while duration of azole therapy for coccidioides meningitis is lifelong. Number of references: 219 — a relatively “low” number, maybe because cocci’s geographic distribution isn’t very wide. Nah, 219 references is still a lot.
- Treatment of drug-susceptible tuberculosis. The winner for the most number of organizations involved in developing and endorsing these guidelines — I counted 9 (just read the start of the abstract). A selected key recommendation: Initial adjunctive corticosteroid therapy should not be routinely used in patients with tuberculous pericarditis. Number of references: 531 — would be top of the pack if you added the other TB Guidelines (which are next).
- Diagnosis of tuberculosis. First update on this topic in 17 years, so long overdue. A selected key recommendation: Use an interferon gamma release assay (IGRA) to assess for latent TB in place of the tuberculin skin test (TST) in most clinical settings. I’m ok with that! Number of references: 241 — hey, it’s just diagnosis after all.
- Leishmaniasis. Diagnosis and treatment of cutaneous, mucocutaneous, and visceral disease. A selected key recommendation: Use a reference laboratory to perform culture and PCR in an effort to identify the infecting parasite to the species level, which may have implications for management. Number of references: 503, which exceeds the number of cases of leishmaniasis I have seen by 498.
That’s 8 Guidelines, and a total of 3,298 references. Hard at work, IDSA!
Of course no listing of IDSA Guidelines these days is complete without the invaluable HCV guidelines, which I’ve praised (and use) often. They’ve changed so frequently since their inception that they have their own special site, and aren’t really “published” anywhere else — at least not in a traditional medical journal. On the plus side, this allows the HCV Guidelines greater flexibility, with modifications on an as-needed basis (three times in 2016 alone) for important changes in the field — a nod to the DHHS HIV Guidelines, which have a similar structure. On the minus side, there’s no real peer review, and I’m sure some of the writers of the guidelines (especially those seeking academic promotion) wouldn’t mind a byline discoverable in PubMed.
And they don’t number their references, so someone else has to count. Kind of like guessing the number of pennies in a big jar to win a prize.
Finally, here are some bears playing with a pink balloon. Just because.
[youtube https://www.youtube.com/watch?v=HzNEna-PETY]