An ongoing dialogue on HIV/AIDS, infectious diseases,
July 19th, 2020
Reaching Out to ID Doctors in COVID-19 Hot Spots — You Must Be Truly Exhausted
 For us ID doctors in most of the northeastern United States (and Chicago and Detroit and some other northern cities), March and April hit us like a giant wave of never-ending calls, pages, emails, and crises.
For us ID doctors in most of the northeastern United States (and Chicago and Detroit and some other northern cities), March and April hit us like a giant wave of never-ending calls, pages, emails, and crises.
With COVID-19 case numbers increasing every day, the challenges crashed down on us in an endless torrent of hospital needs and responsibilities.
The emotional toll of seeing so many critically ill patients for whom we didn’t have any proven therapies was only part of it. We were also the group to whom everyone turned for help — everyone had questions.
We know you must be swamped right now, but … started many a query.
No surprise. This is an Infectious Disease, after all. “It’s what you signed up for,” as one of my friends, a lawyer, put it succinctly with a shrug.
But it’s also something none of us had ever seen in any of our lifetimes. No one is old enough to remember the 1918 influenza pandemic. No one really knows what to do, or how to do it.
Which is why the work of handling COVID-19 can be endless.
Want an example? Someone tallied what one of my extraordinary colleagues — she ran our “biothreats” response team — gave to the COVID-19 effort, which started for her in early March:
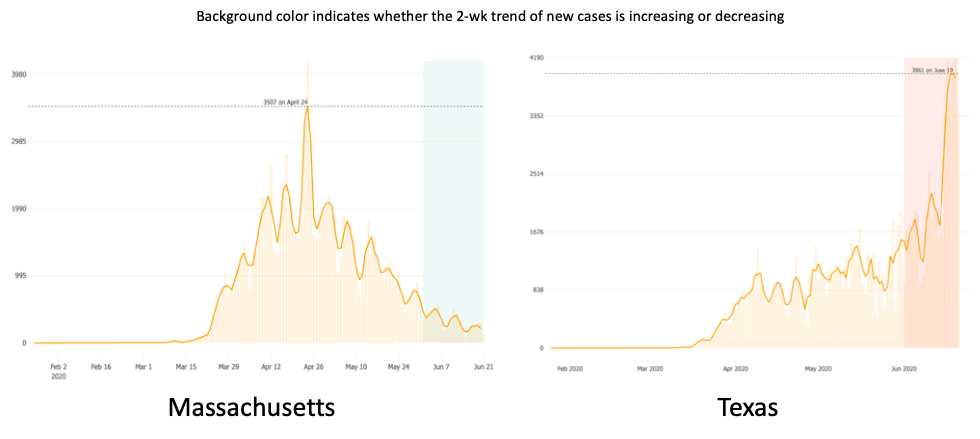
Needless to say, it came as an enormous relief when case numbers started to drop. For Boston, this started in early May.
Here’s what Dr. Stephen Smith, writing in late June, said about his experience in northern New Jersey (reprinted with permission):
In my private ID practice in northern NJ, 18 miles west of New York City, we have treated over 200 hospitalized COVID-19 patients, many in the ICU. We peaked in early April. Then in early May, it was as if someone turned off the spigot. Since then, we have been consulted on only 20 patients who tested positive for SARS-CoV-2, but only a minority was admitted with COVID-19 symptoms. We have not intubated a patient with COVID since early May.
We are hesitant to use the “A” word (attenuation). Like the reverse of saying Lord Voldemort’s name, we are worried that if we say the “A” word, it won’t happen.
To what do we owe this lessening of the COVID-19 burden, especially now that cases are increasing in most of the rest of the country?
While herd immunity might play some role — I remain hopeful, despite discouraging reports on low seropositivity in hot spots — much more likely is that we’ve been adherent on a societal level with implementation of various low-tech strategies.
Social distancing. Banning communal indoor activities. Closing bars and restaurants. Asking religious institutions to worship over Zoom, or outside. Wearing masks in public, especially indoors.
These things, you know, actually work.
While we ID doctors in the northeast bask in this much-needed reprieve, our ID colleagues in much of the rest of the country — initially relatively spared — now have to deal with rising case numbers.
And they must be truly exhausted. Really.
Because it’s not as if they weren’t gearing up for this in the winter. When CROI (the most important national HIV meeting) converted to a “virtual” meeting in early March, many ID doctors from the south and west had already cancelled their plans to come to Boston — COVID-19 already loomed very large nationally to all us ID specialists, and preparation for it was underway everywhere.
Plus, note I wrote that they were relatively spared — they’ve also been seeing some cases right from the start, if not as many we initially did.
So to all ID docs out there in Arizona, Florida, Texas, California, Alabama, South Carolina, Tennessee, Georgia, Louisiana, and all the other states experiencing a COVID-19 surge, I’m thinking of you.
And so hoping that your community will get it together, and turn off that spigot soon.
Because when it happens, it brings a relief the likes of which you won’t believe.
July 15th, 2020
Really Rapid Review — AIDS 2020 Virtual
The International AIDS Conference — or AIDS 2020 — shifted from its Bay Area dual locations of San Francisco and Oakland to be entirely online.
Digital. In the cloud. Virtual.
The primary motivation for the switch was to show off what the numerous tech giants in the region could do with this fancy thing called the World Wide Web.
You know — Google, Apple, Facebook, Oracle, Intel, Hewlett-Packard, Cisco, Twitter, Zoom, and the whole gang — flexing their digital muscles.
Ha, ha.
With that silly preface out of the way, here’s a Really Rapid Review™ of some highlights — which encouragingly included some very important HIV research. Off we go with some highlights:
- Long-acting injectable cabotegravir is superior to TDF/FTC for pre-exposure prophylaxis in MSM and transgender women. Sure, we’ve known some of these results of HPTN 083 for ages — that’s May 2020, isn’t time strange these days? But seeing the data makes the results even more impressive, especially since 1) the study enrolled the demographics at highest risk for HIV; 2) both strategies were highly effective, CAB just more so; and 3) based on when incident HIV occurred, it looks like only 5 out of 2200 acquired HIV while actually receiving injectable cabotegravir. That’s an incredibly small number, regardless of what subsequent resistance studies show. Those are strangely not yet available — blame COVID-19.
- People in Boston stopped PrEP due to COVID-19. Completely understandable — why take PrEP if you’re social distancing? Similar COVID-19 effect in Australia. Some things definitely cannot happen with a 6-foot space between you and another person. And the virtual version of this activity does not require PrEP — unless those tech giants mentioned above have figured out something very novel. Jokes aside, this trend will bear watching as COVID-19 recedes in some areas.
- The occurrence of neural tube defects in babies born to mothers receiving dolutegravir at conception continues to drop. From an estimate of 1/100 exposures after the first report 2 years ago, to 2/1000 now, this decline suggests that the initial case cluster (n=4) may have occurred by chance. Good news! Note that the difference in neural tube defects between dolutegravir and other ART exposure at conception is no longer statistically significant.
- Despite a faster viral load decline, DTG-based regimens in pregnancy do not appear to prevent maternal-to-child transmission better than treatment with EFV. An unexpected finding is that among the 1074 pregnancies, there were 5 transmissions to the newborn — and all occurred in dolutegravir-treated moms. Though not statistically significant, this numeric imbalance is something worth watching as tenofovir-lamivudine-dolutegravir rolls out globally.
- Treatment-related obesity may increase the risk of adverse pregnancy and infant outcomes, especially with TAF/FTC + DTG. This modeling study used incident obesity from the ADVANCE trial, where 14% of women on this regimen developed obesity at 96 weeks, to predict the occurrence of varous complications. Examples include gestational diabetes, preeclampsia, large for gestational age, and macrosomia (among others). Note that these adverse outcomes were not seen in the IMPAACT 2010 study, which tested this exact regimen in women starting HIV treatment during pregnancy — and conversely found TAF/FTC + DTG to be the safest treatment.
- In South Africa, people with HIV with COVID-19 experienced a twofold increased risk for death compared to those without HIV. As I noted before, this giant study contrasts with most U.S. and European cohorts both in its sheer size — 4016 people with HIV, if I did my math right — and conclusions about the negative impact of HIV on outcome. An important unmeasured contributor is likely to be social determinants of health — especially poverty.
- A cohort study of 100 people with HIV in the Bronx showed no effect of HIV on mortality. This reassuring result is much more typical of the European and U.S. studies of HIV and COVID-19. An interesting observation is that none of the viremic people with HIV required intubation, further suggesting that immune responses to the virus contribute to the most severe outcomes.
- In this VA cohort study, people with HIV were no more likely to test positive for COVID-19 than HIV-negative controls. Disease outcomes similar as well. Results imply that HIV does not predispose to acquiring COVID-19, nor worsens severity of disease.
- Could the HCV drugs sofosbuvir and daclatasvir be effective treatment for COVID-19? Pooled results from three studies in Iran demonstrated a faster recovery time in those receiving these drugs. Results should be considered hypothesis-generating rather than definitive given the small number of patients treated; a 600-person randomized trial is ongoing. If effective, let’s hope the price comes down, as the price of 14 days of this combination in the USA is nearly $19,000.
- One person (out of five) who intensified treatment of with maraviroc, dolutegravir, and nicotinamide (?) is now off therapy with no viral rebound. His baseline regimen was TDF/FTC/EFV, and he’s been off treatment for 66 weeks; HIV antibody titers are also declining. Could this be the next HIV cure? Or just a very small reservoir, potentially primed to rebound, as in the “Boston” patients after stem cell transplants? Only time will tell.
- In a retrospective study of first-line failure with TDF/FTC and an NNRTI, an strategy of recycling tenofovir versus switching to zidovudine favors the tenofovir approach. Note that this is despite a high proportion of K65R resistance mutations among viral isolates after first-line failure. The results were attributed to better adherence on the TDF, which is much better tolerated than ZDV. Agree 100%, and worth studying prospectively since they contradict WHO guidelines.
- In NAMSAL study, DTG remains non-inferior to EFV at 96 weeks. The importance of this clinical trial, conducted in Cameroon, is that it included a very high proportion of participants with high viral loads (66% > 100K) and/or low CD4-cell counts (33% < 200). DTG treated patients experienced less emergent resistance, but EFV did surprisingly well given what is considered a region with high transmitted NNRTI resistance. Both strategies did worse in the high viral load stratum — something I’d expect we’d see more often if our other treatment-naive studies enrolled a comparable population.
- In the NIX-TB study of bedaquiline, pretomanid, and linezolid for drug-resistant TB, HIV status did not worsen outcomes. The most important toxicity of this regimen — so transformative for treatment of such a difficult infection — is linezolid-related neuropathy.
- In a longitudinal clinic-based analysis, a switch from TDF to TAF leads to a 9-month period of weight gain (1–4 kg). Change in weight then resumes at roughly the same slope as before the switch. Whether this is due to the weight suppressive effects of TDF versus a direct effect of TAF — or both — remains an unanswered question. In support of the former hypothesis, weight change in the HPTN 083 study was less in the TDF/FTC than the CAB arm, just as it was versus the placebo arm of iPrEx and TAF/FTC arm of DISCOVER.
- People with HIV starting treatment gain more weight over time than matched HIV negative controls. This large comparative cohort study from Kaiser demonstrates both a “return to health” phenomenon for those starting treatment with low body weight, and an eventual “overshoot” for those with normal or above-normal weight at baseline.
- Metabolic parameters improved when switching from TAF-based regimens to DTG/3TC in the TANGO study. Importantly, a high proportion of the enrolled participants were receiving “boosted” regimens, mostly elvitegravir/cobicistat/FTC/TAF — the bulk of the benefit came from these switches. No significant differences in weight.
- Standard risk calculators appear to underestimate cardiovascular risk in people with HIV. Data derived from two large clinical databases, one from Kaiser and the other from our health system — which has been newly named “Mass General Brigham” (R.I.P., Partners).
- Patients switched to DTG/3TC did not experience more low-level viremia than those remaining on a three-drug TAF-based regimen. Another analysis from TANGO, this time using “target not detected” as the endpoint of interest.
- People with NRTI resistance do not have more more “blips” if suppressed on BIC/FTC/TAF or DTG plus FTC/TAF. Guess if you’re suppressed on these high resistance-barrier integrase inhibitors, you’re really suppressed.
- Participants with virologic failure on doravirine plus islatravir as initial therapy experienced only low-level viremia. In these data from the Phase 2 study, all confirmatory viral load values were <80, so none met criteria for resistance testing. The presentation included a table with the research plans for this DOR/ISL combination, which includes fully powered studies in treatment-naive patients (vs. BIC/FTC/TAF) and switch studies.
- Among NNRTIs, doravirine was most likely to be active against over 4000 viral isolates sent for HIV drug resistance testing. At a biologic cut-off of threefold change, 92.5% of the isolates retained doravirine susceptibility, including 45–62% of those resistant to other NNRTIs. Isolates with single mutations (e.g., K103N or Y181C or V106I) generally remained susceptible.
- The pharmacokinetics of subcutaneous injections of lenacapavir support an every 6-month dosing interval. Introducing a new antiretroviral name for this investigational CAPsid inhibitor (note the third syllable)! A key question is what partner should accompany such an infrequent administration, and at what intervals.
- There is no significant drug interaction between darunavir/ritonavir and dolutegravir. Good thing, too — it’s a very commonly used combination in patients with resistance.
- Among those older than 65, a switch to BIC/FTC/TAF maintains viral suppression and is well tolerated. Raises my spirits to see the age threshold for “older” people with HIV to be moved up from 50 to 65!
- A compassionate use program offers injectable cabotegravir plus rilpivirine to people who cannot or will not take oral antiretrovirals. Of the 35 patients in this program, 28 were viremic — and hence received CAB/RPV treatment not as a switch-strategy, which is how it will be licensed. Still, a remarkable 63% of the total population achieved viral suppression despite enormous challenges.
At this point in these Really Rapid Reviews™, I often comment a bit on the host city’s cuisine, or sites, or weather, or something local. (Sharon Lewin says I captured Melbourne perfectly. Very proud of that comment.)
But since this was a virtual meeting, what can I discuss? The conference website? Acknowledging that it must be a huge challenge to put these meetings on this way, I confess to finding the AIDS 2020 site rather baffling. Fortunately, several people (including one of the conference chairs, Monica Gandhi) circulated brief tutorials. Here’s a nice guide, too.
Still, the search function was wonky, and I couldn’t figure out a way to share the URLs from the abstracts; as a result, the links in the above summary refer to outside sources.
And yes, it’s mostly NATAP, which remains the most reliable place to find conference results — despite a web design that hasn’t been updated since 1999 (my estimate). Thank you, Jules Levin and Mark Mascolini!
As usual, if I missed something important, put it in the comments section. Also — will we meet in person next year in Berlin? What do you think?
June 28th, 2020
Is COVID-19 Different in People with HIV?
From the start of the COVID-19 pandemic, one of most common questions I’ve received has been whether COVID-19 has different clinical manifestations in people with HIV.
Would it be more lethal since people with HIV have impaired immune systems? Or milder since some of the damage in severe cases is immunologically mediated?
Or would it be similar, since antiretroviral therapy (ART) is so effective?
Or maybe people with HIV are protected, as they are already taking antiviral medications — some of which (in particular the nucleoside reverse transcriptase inhibitors, NRTIs) have activity against SARS-CoV-2 in experimental systems.
The honest answer, as it has been for so much of this new disease, is that we don’t know definitively — but the early evidence from small studies suggested COVID-19 was quite similar in those with and without HIV.
This passed the anecdotal test too. Here in Boston, all of us HIV/ID specialists have had patients with HIV develop COVID-19. Absent other medical problems known to be associated with poor outcome, they mostly did fine.
What we need, of course, are larger, well-conducted cohort studies to confirm these impressions. And those are just starting to appear.
In the Annals of Internal Medicine, Spanish researchers describe the incidence of COVID-19 among 77,590 HIV-positive persons receiving ART, looking also at their risk of hospitalization. They primarily focused on their antiretroviral therapy — in particular, the NRTIs.
During a 3-month period, 236 people with HIV were diagnosed with COVID-19, and 151 were hospitalized. The risk of hospitalization by NRTI treatment per 10,000 patients was lowest for TDF/FTC (10.5), while other NRTI strategies were similar (TAF/FTC 20.3, ABC/3TC 23.4, single or no NRTI 20.0). None of those on TDF/FTC died or were admitted to the ICU. The group receiving TDF/FTC also had a lower overall incidence of infection.
What might explain this apparent protective effect of TDF/FTC? As noted above, and further elaborated upon in the paper’s discussion section, NRTIs demonstrate in vitro antiviral activity against SARS-CoV-2; furthermore, tenofovir may have beneficial immunomodulatory effects. The higher plasma and extracellular concentrations of tenofovir DF over tenofovir AF could explain the differences between the two.
But before we leap to that conclusion, remember that similar in vitro observations exist for numerous anti-infective compounds, from A-Z and beyond. (Remember hydroxychloroquine? Oh yeah, that was a thing.) Citing these mechanistic explanations hardly proves that they do anything in human beings.
And of course the people remaining on TDF/FTC today are least likely to have many of the medical comorbidities associated with worse outcome in COVID-19 — they’re healthier at baseline. Most older people with HIV, in particular those with renal or cardiovascular disease, now receive either TAF/FTC, or increasingly, a regimen that does not include either tenofovir or abacavir. The paper does not include data on these factors.
So consider the data from this fascinating paper to be hypothesis-generating rather than conclusive — as readily acknowledged by my long-time friend and HIV/ID colleague from Spain, Dr. Jose Arribas, also a co-author on the study.
We await confirmatory observations elsewhere, perhaps in people currently taking TDF/FTC for HIV PrEP — could it amazingly also be preventing a second viral infection? — or even better, from the results of a pre-exposure prophylaxis randomized study, now ongoing.
Of course these data about the NRTIs don’t get at the original question posed at the start of this post — do people with HIV who get COVID-19 do better, worse, or the same as those without HIV?
Tucked away in the discussion section is this sentence:
In line with the greater all-cause mortality of HIV-positive persons compared with the general Spanish population, we found greater age- and sex-standardized mortality from COVID-19 in HIV-positive persons (3.7 per 10 000 compared) than in the general population (2.1 per 10 000).
A large South African study, furthermore, recently reported the following:
Large study of clinical outcomes of #COVID19 in South Africa demonstrates HIV associated with increased risk of death. Effect has not been seen in smaller European, USA cohorts thus far. No diff by viral suppression. H/T @AntonPozniak https://t.co/TXwlJzsOnQ pic.twitter.com/FUxz5J7Aog
— Paul Sax (@PaulSaxMD) June 17, 2020
The linked slide presentation contains scant data about the HIV population — only that this effect was seen in both those with and without HIV viral suppression. Apparently we’ll hear more at the 23rd International AIDS Conference in July.
So what can explain these apparently negative outcomes of COVID-19 in people with HIV, both in Spain and South Africa?
In Spain and other developed countries, I’m betting on comorbidities — hinted at by the senior author of the Spanish study.
A significant fraction of our older HIV population endured years of uncontrolled viremia, immunosuppression, and toxic ART. This group experiences excess non-HIV medical problems comparable to HIV-negative people who are 5-10 years older. Many of these issues are well-defined risk factors for severe COVID-19, and perhaps controlling for these conditions will yield a prognosis comparable to those without HIV.
For South Africa, additionally, people with HIV tend to come from much more socially disadvantaged backgrounds — it is strongly associated with poverty and lack of access to care, also negative predictive markers in COVID-19 worldwide.
So while data from these larger studies are welcome, we eagerly await more. And in the meantime, we can contemplate what all the acronyms stand for in the alphabet soup that is Jose’s twitter profile.
June 21st, 2020
Dexamethasone Improves Survival in COVID-19 — Why This Should Be Practice Changing Even Before the Paper is Published
When the news broke last week that the dexamethasone component of the RECOVERY randomized clinical trial was halted because those receiving the drug were significantly more likely to survive, I posted the following:
– Very welcome news, dex is cheap, widely available!
– Demonstrates the power of RCTs vs obs studies, which were conflicting
– How will the numerous ongoing studies of immunomodulators be modified?
– Rx guidelines — act now or wait for more info?https://t.co/qBsaZ1csH2— Paul Sax (@PaulSaxMD) June 16, 2020
Note my last point, about “guidelines”. These committees have a responsibility to get what they recommend right, and might be slower than clinicians to recommend an intervention with limited information — even if it is potentially life-saving.
But my assumption was that clinical practice would change quickly, awaiting the updating of guidelines. After all, this is what we’ve been waiting for — data from a randomized trial demonstrating a clear benefit. Even better, it’s a readily available, inexpensive strategy — a course of corticosteroids — familiar to us all.
I confess the responses to my post, and comments elsewhere, surprised me. Lots of skepticism. Wow.
The comments fell into several interrelated categories:
Let’s wait for the study to be peer-reviewed and published in an established medical journal before changing clinical practice.
Really? Even when the sickest patients — those requiring oxygen or ventilatory support — were more likely to survive?
(Yes, I keep italicizing that endpoint. Emphasis, you know.)
For the record, here are the results:
Dexamethasone reduced deaths by one-third in ventilated patients (rate ratio 0.65 [95% confidence interval 0.48 to 0.88]; p=0.0003) and by one fifth in other patients receiving oxygen only (0.80 [0.67 to 0.96]; p=0.0021).
When a study stops because of a survival benefit for a life-threatening disease, take note. It’s because continuing the study as originally designed is unethical — those randomized to receive “usual care” would be deprived of a potentially life-saving treatment.
The steering committee has a responsibility of ensuring the safety of trial participants. And remember, they have access to all the study data, even if we don’t.
It’s critical that this information be made available as soon as possible. Patients are being treated today who might benefit, and writing papers and subsequent peer review take time — typically weeks, even with the “warp speed” of COVID-19.
To quote one of the investigators: “Dexamethasone is inexpensive, on the shelf, and can be used immediately to save lives worldwide.”
Well said.
Why are we getting critical information via press release? I’m inherently distrustful. A press release doesn’t represent actual data.
It’s reasonable to be skeptical of clinical trial press releases, especially when issued by private pharmaceutical companies with multi-million dollar marketing divisions.
These notoriously exaggerate the importance of study results, especially when focused on surrogate markers of disease that may or may not predict clinical outcome.
But consider — this isn’t a press release by a giant company, citing a minor change in an inflammatory cytokine or quality-of-life metric in an open-label study. It’s a respected clinical trials group, funded by the government of Great Britain, and they are reporting a survival benefit from their clinical trial.
To their credit, they early on started doing randomized trials of various COVID-19 interventions while the rest of the globe practiced the therapeutic equivalent of throwing drugs against the wall hoping some of them would stick.
Lopinavir-ritonavir! Interferons! Oseltamivir! Hydroxychloroquine! Azithromycin! Ivermectin!
And it’s not just antimicrobials — virtually every immunomodulator under the sun, some extremely expensive, has found its way to off-label use for critically ill patients with COVID-19. Tocilizumab! Sarilumab! Anakinra! Ruxolitinib! Eculizumab! Any-other-mab! And more …
Yes, it’s hard to keep up — see Table 1 in this recent review for all the various anti-inflammatory approaches tried off-label, with many of these now under study.
If we’re using some of these unproven therapies — and many of us have — why not dexamethasone, which in the RECOVERY trial improved survival?
Here we go again! Haven’t we been burned already multiple times with research on COVID-19, only later to have this information questioned, or retracted?
Quite reasonable to be cautious in this very fast-moving area.
But the infamous research that has “burned” us involved much weaker levels of evidence — little more than anecdotal observations at one extreme and observational studies with likely falsified data at the other.
None has been a randomized clinical trial with a survival benefit.
(Have I noted that result enough times already? Nah.)
I need more details about the study. What were the primary endpoints? The specifics of the intervention? What were the patient characteristics of those enrolled? Did some subgroups benefit more than others? What were the toxicities?
All very reasonable questions! But good news — we have the full protocol available for review. This can answer some of these queries, including the endpoints and description of the exact interventions studied.
It’s a highly valuable document that may allay some concerns that the investigators somehow didn’t conduct the study or analyze the data properly.
And I share the interest in seeing the fully published paper to examine the study results in more detail.
But until it is published, we have now highly favorable results in the most important clinical endpoint in any interventional study — improved survival.
So aside from those patients for whom corticosteroids would be contraindicated, it’s hard to imagine not offering dexamethasone — today — to a person with COVID-19 that requires supplemental oxygen or ventilatory support. They might live longer!
And that’s good news.
Just like getting a fresh video from the sporting archives of Olive and Mabel.
[Originally this post cited the action of a Data Safety Monitoring Board; rather the study Steering Committee assessed that enough patients had been enrolled to answer the question of whether dexamethasone provides benefit. Have modified to reflect this difference.]
June 7th, 2020
Hydroxychloroquine Not Effective in Preventing COVID-19 — In Praise of a Negative Clinical Trial
The headlines might read, Malaria Drug Ineffective in Preventing COVID-19 — but that doesn’t do justice to a remarkable clinical trial, just published this week in the New England Journal of Medicine.
Led by Dr. David Boulware at the University of Minnesota, the study asked this question: Does hydroxychloroquine (HCQ) prevent the development of COVID-19 in people after significant healthcare or household exposure to the disease?
To say that the recruitment of this post-exposure prophylaxis trial was innovative hardly gives the methods enough credit. I first heard about the study in mid-March, based on this post by the lead author:
Healthcare worker exposed to COVID-19? Sharing a home with someone with COVID-19? Our Univ. of Minnesota team at the has launched a clinical trial studying a drug that may help prevent infection in those exposed to coronavirus. Email us at covid19@umn.edu to enroll.#IDTwitter pic.twitter.com/eFa425j2Z5
— David Boulware, MD MPH (@boulware_dr) March 17, 2020
It wasn’t long after reading this that I received a frantic message from one of my long-term patients with just this sort of high-risk exposure. I immediately referred him to the study.
With this study design, he didn’t have to fly to Minnesota to enroll — it was all done remotely. Informed consent, medication dispensing and shipment, adverse event monitoring, endpoint assessments.
(He did fine, by the way.)
In essence, this randomized, placebo-controlled study gives new meaning to the phrase “multicenter clinical trial”! I count 821 “centers” — specifically, the homes of the 821 participants, 414 of whom received HCQ, 407 placebo.
As the authors note, the conduct of the study had major advantages:
This approach allowed for recruitment across North America, minimized the risk of SARS-CoV-2 infection to researchers, lowered the burden of research participation, and provided a timely answer to this question of whether postexposure prophylaxis was effective. Moreover, this approach allowed broad geographic participation regardless of anyone’s physical distance from academic centers, increasing the generalizability of the findings.
Think also of the countless time, money, and effort saved by avoiding the need for local institutional review board approvals, clinical trials contracts, and study visits. Remarkable.
No, this isn’t the first study to enroll and follow participants remotely — the groundbreaking Physicians‘ and Nurses’ Health Studies come to mind. But it’s certainly the first one conducted and completed during a global pandemic.
For the record, the incidence of clinical COVID-19 illness did not differ significantly between groups (11.8% for HCQ, 14.3% placebo), and people receiving HCQ had more side effects, mostly gastrointestinal. There were no serious cardiac adverse events, a problem reported on other studies (especially when combined with azithromycin).
As noted in the accompanying editorial (and acknowledged by the authors), the study had several limitations, most notably the small proportion of COVID-19 cases (just over 10%) confirmed by PCR, and the delay (3 or more days) between exposure and starting preventive treatment. Furthermore, such a remotely conducted study cannot collect highly detailed data.
These and other limitations mean that these results may not be definitive; other prevention studies with HCQ continue.
Still, kudos to the investigators for so quickly carrying out this remarkable study. It’s a reminder that sometimes the innovation in clinical trials comes in the methods section, not in the results.
May 31st, 2020
America the Not So Beautiful Right Now, with a Must-Read Book Suggestion
Have you read W. Kamau Bell’s The Awkward Thoughts of W. Kamau Bell: Tales of a 6′ 4″, African American, Heterosexual, Cisgender, Left-Leaning, Asthmatic, Black and Proud Blerd, Mama’s Boy, Dad, and Stand-Up Comedian?
If you haven’t, may I suggest you put it at the top of your list, and pronto? In addition to being funny, moving, and thought-provoking, it’s one of the most influential books I’ve read in the past decade — and could not be more relevant now.
And by influential, I mean influential to me — a white guy living in the United States, and therefore generally oblivious to the terrifying racism blacks experience here on a regular basis.
So probably influential to anyone who hasn’t experienced what he describes in this brilliant book. It acts as a great antidote to our complacent cluelessness.
There’s a chapter I’ve thought about so many times since, especially this week. It’s called, “Awkward Thoughts about Being a Black Male, Six Foot Four Inches Tall in America”.
Here’s how the chapter starts — apologies for the lengthy quote, please read to the end:
I am afraid of the cops. Absolutely petrified of the cops. Now understand, I’ve never been arrested or held for questioning. I’ve never been told that I “fit the description.” But that doesn’t change a thing. I am afraid of cops the way that spiders are afraid of boots. You’re walking along, minding your own business, and SQUISH! You’re dead. Simply put, I am afraid of the cops because I am Black. To raise the stakes even further, I am male. And to go all in on this pot of fear, I am six foot four and weigh 250 pounds . . . at least. (I stopped keeping track, which is the next best thing to actually working out.) Michael Brown, the unarmed Ferguson, Missouri, eighteen-year-old shot dead by police in the summer of 2014 , was also six foot four. Eric Garner, who was strangled to death by the NYPD, was six foot three. Depending on your perspective, I could be described as a “gentle giant,” the way that teachers described Brown and the way that friends described Eric Garner. Or I could be described as a “demon,” the way that Officer Darren Wilson described Michael Brown in his grand jury testimony. And just like Eric Garner, I have asthma, so when I hear him say on video, “I can’t breathe,” as the officer chokes the life out of him for selling loose cigarettes, I can feel it in my chest as I’m sure he did.
Oh that last sentence — so chilling. Written in 2017, in case you were wondering.
Some readers might wonder why I’m covering this topic today here on an ID blog. Some might suggest I stick to medical topics, say they don’t come here for this kind of commentary, accuse me of “virtue signaling”.
To them I say — feel free not to read this. No one’s forcing you.
And imagine if I didn’t comment. So much worse — unbearably so, at least for me. Our country’s history of racism is so long and painful the problem will only improve if we all fight it together.
Meanwhile, if you can’t stand a post without ID, how about this from super ID fellow Dr. Aaron Richterman, commenting on the staggeringly disproportionate toll COVID-19 has taken on people of color?
Remarkable that this paper in @NEJM alludes to genetic differences but doesn't mention the word "racism" one time.https://t.co/SetGVpnfU1
— Aaron Richterman, MD (@AaronRichterman) May 27, 2020
Highly relevant.
May 25th, 2020
A Major Advance in Non-COVID-19 ID Research You Might Have Missed
 One thing about the COVID-19 pandemic — other important non-COVID ID news gets crowded out.
One thing about the COVID-19 pandemic — other important non-COVID ID news gets crowded out.
As a prime example, take HPTN 083, a major clinical trial in HIV prevention. The results are a big deal, and should have garnered more attention when they were released last week.
This randomized, double-blind pre-exposure prophylaxis (PrEP) study compared long-acting injectable cabotegravir (CAB), given every 2 months, with a control arm of daily TDF/FTC (brand name Truvada). The population was men who have sex with men and transgender women at high risk for HIV. The National Institute of Allergy and Infectious Diseases funded the study with the drug manufacturers (ViiV and Gilead, respectively) providing the study drugs.
In reviewing the results of the ongoing trial, a data safety and monitoring board (DSMB) found the data so compelling that they stopped the blinding and informed participants of their treatment assignment.
DSMB actions like this are generally big news — the same thing happened recently with the COVID-19 remdesivir study comparing it to placebo, showing a faster recovery time in the remdesivir arm. So let’s take a brief break from all-things-COVID and look in more detail at the HPTN 083 results.
The study enrolled 4570 participants with a mean age of 28 years; 12% were transgender women. Importantly, 50% of the U.S. enrollment identified as Black or African American, the group in this country at disproportionately high risk of HIV. Participants also came from Argentina, Brazil, Peru, Thailand, Vietnam, and South Africa.
During the study, 50 participants acquired HIV, for an overall incidence rate of 0.79%. Since the investigators predicted a background HIV incidence of 4.5%, these results show that both study arms were highly effective in preventing HIV.
But here’s where it gets really interesting — 38 of the infections came in the TDF/FTC arm (incidence, 1.21%), versus only 12 (incidence, 0.38%) in the CAB treatment group.
Let’s do the complicated math — there were about three times fewer HIV infections acquired by participants on CAB than on TDF/FTC.
Since there’s still no baseball, I’ll fill the gap by applying the appropriate metaphor — this most certainly is a home run for HIV prevention. And, if you prefer Greek epic poetry metaphors, it appears that injectable cabotegravir overcomes the Achilles heel of daily TDF/FTC, which is adherence.
Safety and tolerability of the two approaches were similar, though injection site reactions were more common in the CAB arm. Remember, though all received active drug, accompanying placebos were also administered — kudos to the study sites for pulling this off, that’s a lot of injections.
The press release stated that resistance testing is “in progress” — these data will be critical. If the failure of CAB leads to integrase resistance, this will be a substantial disadvantage to this otherwise very promising strategy.
An additional endpoint of some interest (also not yet reported) will be weight changes over time since TDF/FTC tends to suppress weight, while most integrase-based regimens in people with HIV lead to weight gain. No effect of cabotegravir on weight was observed in a small previous study, but there was no TDF/FTC control arm.
Importantly, HPTN 084, a parallel trial designed to evaluate CAB in comparison to daily oral TDF/FTC in women in sub-Saharan Africa, is ongoing. The results of both these studies will capture the populations at greatest risk of HIV globally, and fully round out our understanding of these two strategies of HIV prevention.
As noted here previously, the FDA delayed approval of long-acting cabotegravir and rilpivirine late last year, so as of today clinicians cannot prescribe cabotegravir for PrEP. But you can be sure these results will add urgency to completing the approval process, COVID-19 notwithstanding.
They also will amplify the importance of other long-acting PrEP studies in development, including the injectable capsid inhibitor GS-6207 and the nucleoside reverse transcriptase translocation inhibitor islatravir, which is also undergoing evaluation as an implant.
Before finishing, I offer this disclosure and a personal note. The protocol chair of HPTN 083 is Dr. Raphael (Raphy) Landovitz, a longtime friend and former colleague mentioned numerous times on this site. I first met him (many) years ago when he was in medical school, and noted him to be brilliant, opinionated, funny — and most emphatically (and refreshingly) not your typical stereotype of the successful academic.
When he left Boston for UCLA to work under the research mentorship of Dr. Judy Currier, the energy level of the country shifted noticeably westward. Our loss. Regardless, it has been enormously gratifying to watch him go from success to success, with HPTN 083 just the latest example.
And even though he and I don’t share hobbies — he’s passionate about Broadway musicals (hard pass for me) but can’t stand baseball — even I have to admit that this is very, very well done:
May 17th, 2020
Does Strictly Limiting Outdoor Activities Help Prevent the Spread of COVID-19? A Call for Reason
I really miss playing tennis.
Tossing that out there to confess up front why the following might not be the world’s most objective perspective.
But take a look at this:

Jeepers, much of that advice strikes me as silly. Or, as put bluntly by one respondent here:
So, if I understand well, I can sleep with my wife but playing tennis with her just crosses the line, right? S**t, this is a tricky virus!
Ok, back to me and tennis. Some years ago, my wife gave me a nice tennis racquet for a certain milestone birthday.
She had just watch me play in a casual pick-up tournament for novice players at a local park, and noticed I couldn’t shut up about how fun it was for weeks.
It was easily one of the best birthday presents in the history of the planet. I’ve been playing tennis 2-3 times a week ever since. While walking, biking, or driving to the courts, I get this delightful giddy feeling of excitement each time.
Plus — and other devoted amateur athletes and hobbyists will certainly recognize this feeling — playing tennis is one of the few activities during which my mind goes blank on work issues. Patient care worries, manuscript deadlines, demands for learning objectives, human resources struggles, required online learning modules, email overload — all gone.
Boy, I miss that.
So I acknowledge some bias here on the question of whether strictly limiting outdoor activities actually limits the spread of COVID-19.
And — must be said — missing tennis is trivial compared to the hardships, health issues, and losses wreaked by this awful virus.
But follow me on the tennis thing anyway, because I do think that some of our most important public health messages get diluted by nonsensical and absolutist guidelines not rooted in science.
Where do we see most of the spread of COVID-19?
Households, especially those that can’t isolate symptomatic individuals due to lack of space. Crowded settings with poor ventilation. Nursing homes. Shelters. Ships. Call centers. Restaurants and bars. Parties. Family gatherings. Public transportation. Meat packing plants.
What do all of these settings have in common? They’re indoors. Or they’re crowded. Or even worse — both.
That’s because the likelihood of getting infected directly correlates with the amount of virus in the air we’re breathing, and the time we spend breathing it. Read this magnificent and widely circulated post by Dr. Erin Bromage, if you want more details.
Or this wonderful summary of carefully done transmission studies by Dr. Muge Cevik:
A lot of discussion recently about transmission dynamics, most of which are extrapolated from viral loads & estimates. What does contact tracing/community testing data tell us about actual probability of #COVID19 transmission(infection rate), high risk environments/age?
[thread]— Muge Cevik (@mugecevik) May 4, 2020
Outbreaks linked to outdoor activities invariably involve crowds — this Italian soccer match, for example.
How about the infamous Spring Break 2020 revelers, basking outdoors in that glorious sunshine while the rest of the country watched, horrified?
It’s possible that they contracted COVID-19 on the beaches, but much more likely these transmissions occurred during the evening hours while packed together like sardines — not just in their shared hotel rooms, but also while attending museums, poetry readings, and chamber music performances.
(Oops, I mean bars, bars, and more bars.)
Again, the problem with too-strict or illogical public health advice is that they make us distrust all the messages. As noted by population scientist Dr. Julia Marcus in this excellent and thoughtful piece:
The choice between staying home indefinitely and returning to business as usual now is a false one. Risk is not binary. And an all-or-nothing approach to disease prevention can have unintended consequences.
Some take-home messages? Yes, avoid crowds. And indoor spaces with poor ventilation. Keep your distance from others when you can — and when you can’t, limit the time spent in close proximity. And wash your hands a lot.
But do go outside and get some fresh air. Don’t yell at the jogger across the street without a mask, or the person having a picnic in the park with their family — they are not going to infect you.
And let’s be reasonable when it comes to public health messages, focusing on what really counts.
Tennis anyone?
May 10th, 2020
Thank You to Inpatient Nurses — The People Doing the Most Direct COVID-19 Patient Care
Anyone who does inpatient medicine or surgery knows well the major imbalance in time spent on direct patient care between doctors and nurses.
Nurses spend way more time actually with patients than we do — I’m referring to time in the rooms caring for patients.
While we round and review charts, document lab test results, bring up radiographic images, write orders, and debate the merits of the latest hydroxychloroquine or remdesivir study, they do direct assessments and respond most immediately to patient needs.
It’s been this way for years. The late Dr. Arnold Relman, former editor of the New England Journal of Medicine, sustained a major injury several years ago that required a prolonged hospitalization. Writing about the experience, he cited how doctors (vs. nurses) practice medicine today:
What I hadn’t appreciated was the extent to which, when there is no emergency, new technologies and electronic record-keeping affect how doctors do their work. Attention to the masses of data generated by laboratory and imaging studies has shifted their focus away from the patient. Doctors now spend more time with their computers than at the bedside. Reading the physicians’ notes in the MGH and Spaulding records, I found only a few brief descriptions of how I felt or looked. Conversations with my physicians were infrequent, brief, and hardly ever reported. What personal care hospitalized patients now get is mostly from nurses.
Emphasis mine.
COVID-19 only brings this stark disparity into sharper focus. A diagnosis of COVID-19 means necessary isolation — single room, no visitors. Clinicians must put on (“don”) personal protective equipment (PPE) to see patients, then take off (“doff”) the PPE without contaminating oneself — a strategy best done with an attentive monitor. It’s not easy to get it right.
Since PPE supply chains have been strained right from the start, we must limit the number of clinicians with direct patient care. Bring on the telephone or iPad consults — that was never a thing before. Way fewer doctors in the room, both during rounds and the rest of the hospital day. But all patients still have the same amount of nursing care.
We ID specialists say this again and again about the risk of transmission of SARS-CoV-2, the cause of COVID-19. The riskiest activities involve spending prolonged time in close proximity to someone with the disease — especially someone who’s acutely ill and coughing.
And in the hospital, the health professionals doing most of this high-risk activity are the inpatient nurses.
I’ve thanked them before for this. But May 6-12 is National Nurses Week — an excellent reminder to be grateful again for what they are doing in this very challenging time.