An ongoing dialogue on HIV/AIDS, infectious diseases,
September 19th, 2012
It’s Time to Dump the HIV Western Blot
Hard to believe, but we have to get rid of the HIV Western blot — at least as our HIV confirmatory test.
Here’s why (case adapted from several seen the past few years; I’m sure most of you have seen similar):
- 30-year-old man, high risk for HIV. He’s worried he might have become infected due to recent exposures, so he requests testing at a community health center.
- The clinician does an oral rapid test, which is positive, then sends a blood sample to the lab for confirmation.
- The ELISA test is also positive, so the lab sends this sample off-site for confirmation by Western blot.
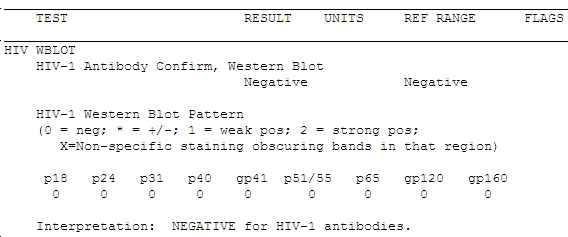
- One week later (the Western blots always take longer than you think they should), the result comes back:
Does that worry you? If not, it should — the guy’s HIV viral load was > 1 million copies/mL. Not only that, but the clinician who did the testing told him that he should return in 6 weeks for a repeat test to see if he fully seroconverts. The patient suspected something might be wrong with that advice (you think?) and sought a second opinion from one of my colleagues, who sent the viral load.
The case highlights what Bernie Branson from the CDC has been telling us for years, which is that the Western blot is lousy at detecting recently acquired HIV. (Great CROI 2012 presentation by Bernie here, when you have a moment.) The Western blot is barely more effective as a “confirmatory” test in this setting than if the sample had been sent for Tropheryma whipplei antibody — and we all know how often those are helpful.
(Little inside ID joke there.)
The fact is that there is a 40-day delay from when “4th Generation” antigen/antibody combination test turns positive to when the Western blot does so. That’s a long time for a person not to know whether he/she has HIV, especially since this is the most contagious period in all of HIV infection.
So what should we do? Chaired by Eric Rosenberg, The Clinical and Laboratory Standards Institute (CLSI) issued a new testing algorithm (M53-A) in July, and the CDC will soon follow with similar recommendations. In essence, the most important algorithm will look like this (figure from Bernie’s excellent 2010 paper):
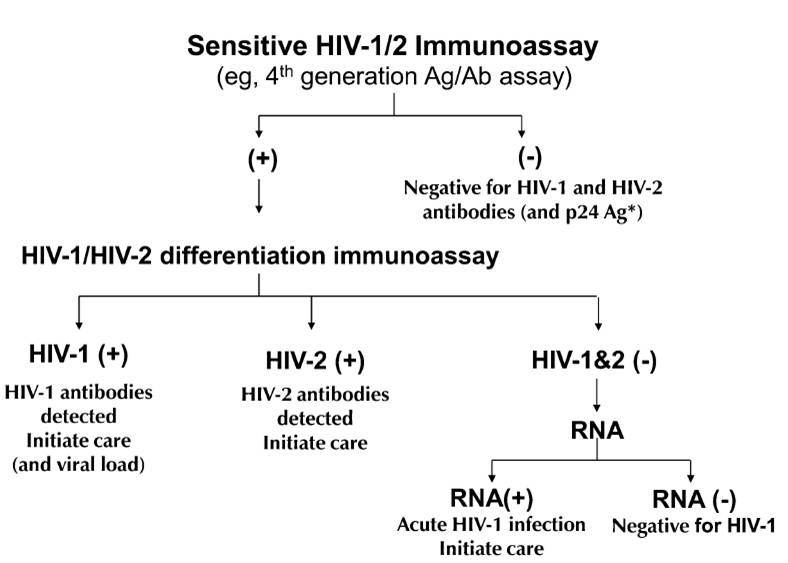
One immediate problem is that most labs don’t have an “HIV-1/HIV-2 differentiation immunoassay”. Only one such test — the Bio-Rad Multispot HIV-1/HIV-2 Rapid test — is FDA approved, although apparently another test is in the late stages of development.
But until such assays are widely available, here’s some practical advice we can give clinicians today:
- Current HIV screening tests (ELISA IgM or ELISA/Ag combinations) are much more sensitive than the Western blot.
- Every case with a positive screening test but a negative Western blot must have an HIV viral load.
- Every case in which HIV acquisition might have been recent — symptoms of acute HIV, or HIV testing in the context of a recent STI (especially syphilis), or known recent negative HIV test — should have an HIV viral load sent along with HIV antibody testing (or at the very least, be tested using a 4th Generation combined antigen/antibody test).
Check out this article from the March 2012 issue of CAP Today for a complete discussion.
And to the HIV Western blot, hey, it’s been great — I thank you for your 20+ years of excellent service! Now it’s time for us to move on.


My main concern about this new algorithm would be a potential loss of specificity, especially in the context of low risk patients. This may result in more false positives.
Indy, agree this is the concern — especially since the Western blot is so famously “specific”, with such low false positive rates. But several things are different today — the ELISA is more accurate, the “differentiation assay” is a second way of assessing antibody, and finally, once HIV antibody is confirmed, we have the HIV viral load test which acts as a completely different way to confirm HIV infection.
For those rare patients who are antibody positive but HIV RNA negative — the so-called “elite controllers” — then the Western blot should still be ordered to confirm HIV infection. But will be a very limited use of a test we now use on every patient!
Paul
If the patient is hailing from an area where HIV prevalence is common or when the patient is having symptoms of HIV like oral thrush, recent sudden loss of weight more than 10% or any other AIDS indicating or defining disease, there is no point in extra confirmation. Most of the rapid and ELISA are 98% specific and sensitive and we are repeating the same at least twice before disclose the status. Some already confirmed by DNA PCR. So there is no point in going for western blot test which costs another extra expense for the patient which is very much concern in a country like India. So I am not go for western blot with my patients unless if there is no history and remain asymptomatic. After knowing the fact that the western blot has no roll in recent acquired infection, I would like to discontinue the same totally. But one should remember that in case of HIV 2 infection or HIV1 and 2 infection, as there is no RNA or DNA test are available freely for HIV2 infection Western blot has a role to confirm that we are dealing with HIV 2 infection and to treat with appropriate ARV drugs.
I’m no expert on this, but why choose a viral load test rather than a qualitative nucleic acid test? Viral load tests are not FDA approved for diagnostic use, while qualitative tests such as the Aptima HIV-1 RNA Qualitative Assay are, and are specifically designed and validated for the purpose, including the identification of early infection.
In your case above the VL reading of 1 million is pretty convincing evidence of acute HIV infection, but viral load assays can sometimes give misleading or ambiguous results – “undetectable” VL in someone who is truly infected, and occasional low readings of 100 or 1000 in uninfected patients.
Western Blot carried out correctly does indeed have a low to negligible false positive rate, but its specificity is actually relatively poor: specificity is not a measure of false positives, but of correctly identified negatives and WB yields a high rate of indeterminates among truly uninfected specimens.
As you point out, its sensitivity is also not that great, especially in the early stages of infection. Given that we’re increasingly focusing on identifying infections as early as possible, I agree that it’s probably not the right tool for the job, given the alternative options.
Colin,
Thanks for your comments.
First, you’ll note the algorithm does not even include HIV RNA as one of the confirmatory steps; it only comes in after two different antibody tests are positive.
Second, there’s only one FDA-approved
quantitativequalitative test, but in fact we don’t know how its false-positive rate and sensitivity compare to the current HIV RNAqualitativequantitative assays. The latter are not FDA-approved for diagnosis because the makers have not done the validation studies. In addition, the HIV RNAqualitativequantitative studies are much more widely available (for example, we run the test in our hospital lab) since they are used much more often for disease monitoring.Third, as for WB specificity, I was referring to the flip-side (which is false positive rate), and as you know the fully positive WB has an unbelievably low false-positive rate (usually it’s due to human error, not a problem with the test).
All in all, we’re in agreement it’s time for the WB to go!
Paul
Interesting. Years ago an HIV viral load cost enormously more than an ELISA plus WB. How much more would this new approach cost? Would this approach be as cost effective in Boston as it would be in Provo, Utah?
Thanks.
Gerard
Gerard,
Yes HIV viral loads cost more — but importantly note that it won’t be necessary in the vast majority of cases when we’re simply doing a screening test. Also, in the proposed algorithm viral load is only indicated when two different antibody tests are positive. This increases the prior probability of true HIV infection enormously, and hence makes the VL a test you’d want to get to stage the patient anyway.
Until that second HIV antibody test is widely available, however, I maintain that if we have anyone with pos ELISA/neg WB, we’ll need to get the viral load test. This isn’t a routine occurrence, but it’s quite common in recently acquired infection and often has significant implications — different from when the ELISA/WB two step testing was originally developed.
Paul
Hi Paul – totally agree with your assessment. I think in your number 5 comment above, “quantitative” and “qualitative” tests were mixed up?
Susan
Thanks, Susan — fixed now!
Paul
Recently I come across a strange experience with Western Blot Test. A sero-discordant couple, husband HIV 1 positive and on ART since 18 months and doing well. Since the husband is an alcoholic, seems not very strict on safe sex practice, I reexamined the wife’s status. She somehow went for a western blot instead of rapid HIV test with a renowned laboratory.
Western blot came as HIV 1 negative and HIV2 positive for her. She denied any short of extramarital affairs. I once again checked the husband blood with HIV combo Triline and tridot test. It came as only HIV1 positive and no evidence of super infection with HIV- 2 infection. Repeating the wife’s blood with ELISA and HIV Combo Triline and Tri dot test reveals she is still maintaining the sero-discordant stage or remained sronegative.
This is another pitfall of western blot with false positive result that I want to bring it to your notice.
The Plex-Id from Abbott should go a long way in making this diagnosis faster and simpler. Hopefully out soon, the only thing it can’t diagnose is prion type diseases. The diagnosis usually in five hours or less. ted bash
will this change the overall testing window period guidelines
Just had a patient like the one described by you.Positive ELISA HIV RNA 370,000 copies, clinically screaming AIDs yet Western blot was negative. VL and clinically doing great on ARVs thanks to our ID consultant.
The link to the article from CAP Today appears to go to a marketing publication with many pages. Are you sure this link is correct? Can you give a page number?
Dear Dr. Sax,
I have had my troubles with the HIV Western Blot as confirmatory test as well. I have repeatedly tested positive to the HIV AB test but have only received negative/inconclusive Western Blots. I have taken PEP and PrEP to try to avoid seroconverting as well. I have always had a undetectable viral load.
Recently, I received the strongly positive Western Blot I was hoping never to get. But when I was re-tested the Western Blot went negative (yet again) and my doctors chalked this false positive up to “lab error.”
Does this mean I am a elite controller/long-term nonprogressor?
Best,
Donald
Donald,
Complex situation — well worth your seeing an ID/HIV specialist to review all the tests. In certain cases, we’ve been able to send specimens to CDC for further work-up if the situation still isn’t clear.
Regards,
Paul
Thank god for HIV RNA tests !! We’re in 2014 people!!
I have followed up with multiple IDs. They seem to believe that I was definitely exposed (as I know I was, since I had condomless contact with an HIV positive person) and that the exposure transmitted some HIV to my body. However, the PEP or PrEP seems to have blocked the exposure from infection “taking hold.”
I am still wary of coming off Truvada as I worry I will seroconvert then. And, since I am still at high risk sexually, I am continuing to use it as PrEP. Once I find a more longterm partner perhaps we will take me off the medication and see what happens. Or perhaps within a few months I will seroconvert. Nevertheless my “in-between” status makes me think that the distinction between HIV-positive and HIV-negative isn’t as clear as most people think.
Dear Dr. Sax,
I am a Brazilian Clinical Pathologist. Our country has just started to implement an algorythm for HIV diagnosis where a positive 4 Gen Ab screening assay is followed by RNA quantitative assay.
The rationale behind this is detecting most of the acute infections when they are still acute infections, ie, on the very same first sample.
I believe there are shortcomings on this scheme, such as despite having a very low HIV incidence, this cases will be missed.
What is your opinion regarding the use of HIV RNA as confirmation assay (before HIV 1 2 Ab differentiation asays)?
Regards
Flavio,
One issue with the algorithm you describe is that HIV-2 cases will be missed, as standard HIV RNA assays are negative. In addition, a small subset of people with HIV-1 will have undetectable HIV RNA — the so-called “elite controllers.” They will have a positive differentiation assay, and hence will be among the few patients who still need an HIV Western blot for diagnosis.
Paul
What is the window period for 4th generation HIV test?