An ongoing dialogue on HIV/AIDS, infectious diseases,
December 13th, 2009
Infection and the ICU: Outcome Predictable, but Important
If you enrolled over 14,000 ICU patients into a study on a single day, and then did follow-up, what would you find regarding the relationship of infection to the outcomes of ICU stay and mortality?
Just such a study was published in JAMA last week, and here are the not-so-stunning conclusions:
Infections are common in patients in contemporary ICUs, and risk of infection increases with duration of ICU stay. In this large cohort, infection was independently associated with an increased risk of hospital death.
To an ID specialist, this is kind of like reading that someone has done a study linking time spent outside in the rain and the likelihood of becoming wet. Patients in ICUs are susceptible to getting infections for innumerable reasons — so many that it seems to us (from our admittedly biased perspective) almost remarkable when an infection doesn’t occur.
In all seriousness, ICU-related infections are a gargantuan problem, and if this study helps publicize the clinical and research needs, more power to it.
December 8th, 2009
Vancouver, Phishing Phlu Scam, Telavancin, and Cartoon
 A few things to ponder as the flu activity (mercifully) declines, at least for now:
A few things to ponder as the flu activity (mercifully) declines, at least for now:
- Interested in evidence that HIV treatment has become staggeringly effective? Fully 87% of patients receiving treatment in the large British Columbia cohort have an HIV RNA < 50; not only that, the incidence of HIV drug resistance has declined more than 12-fold since 1997. Wow. I wouldn’t want to be in the business of selling resistance tests.
- Did you read about this scam? The text from the hoax is pretty awkward — “This profile has to be created both for the vaccinated people and the non-vaccinated ones” is a choice bit of prose — but one could easily imagine how it could trap at least somebody. And as an example of the levels to which humans will stoop to make money, using fear of a real virus to spread a computer one has to be way down there.
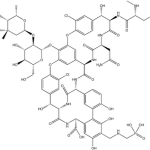
- Has anyone yet actually used telavancin? Not in a clinical trial, but in a patient in clinical practice? If so, what were the indications? How did it go?
- Related, here is a great cartoon — but there’s an even better one on antibiotics that has not yet appeared on-line at The Cartoon Bank, so stay tuned.
December 2nd, 2009
So Much in Less than a Week!
 First the updated WHO Guidelines. Then the following:
First the updated WHO Guidelines. Then the following:
- Updated DHHS Guidelines. Agree? Disagree? Sensible or crazy? Practical or ivory-tower academic?
- South Africa does the right thing. Yes, it’s about time, but good news nonetheless.
- 2012 International AIDS Meeting in Washington, D.C. First time in USA in a long, long time — 1990, to be exact — and possible now that entry restrictions on people living with HIV will be dropped in January 2010. So mark your calendars now, and make sure you dress light for the weather.
Why so much at once? World AIDS Day? Phase of the moon? Or just chance?
November 28th, 2009
ICAAC-IDSA — Alone Again (Naturally)
 Just received my latest copy of Infectious Disease News, that large glossy review magazine* that arrives approximately monthly in my mailbox.
Just received my latest copy of Infectious Disease News, that large glossy review magazine* that arrives approximately monthly in my mailbox.
As usual, I turned right to Dr. Theodore C. Eickhoff’s always-thoughtful editorial, this month entitled “Reflections on the 47th IDSA Meeting.” He writes:
It was a much more “user-friendly” number of attendees, in contrast to the almost 15,000 people that attended the joint meeting last year. I heard absolutely no one express a desire to have another joint IDSA-ICAAC meeting.
I can see his point — the combined meeting in Washington last year drew over 15,000 participants, and at times was just too gargantuan to manage.
But if I could for a moment be the lone voice arguing for a joint meeting, here’s my pitch: Last year, tons of people in ID went to ICAAC-IDSA, even people who rarely go to either meeting. This year, not so much — which might explain the “somewhat muted and subdued” tone Eickhoff found at IDSA this year in Philadelphia.
Either that or what the Yankees were doing against the Phillies.
(*Don’t call it a “throwaway!”)
November 20th, 2009
Ties Tied to Bugs
 Are doctors’ neckties causing infections? That’s the implication of this Wall Street Journal piece:
Are doctors’ neckties causing infections? That’s the implication of this Wall Street Journal piece:
The list of things to avoid during flu season includes crowded buses, hospitals and handshakes. Consider adding this: your doctor’s necktie. … A 2004 analysis of neckties worn by 42 doctors and medical staffers at the New York Hospital Medical Center of Queens found that nearly half carried bacteria that could cause illnesses such as pneumonia and blood infections. That compared with 10% for ties worn by security guards at the hospital.
This is old news, of course (yet somehow it warranted front page coverage in the WSJ, go figure). In fact, the British went so far as to ban neckties for doctors entirely in 2006, stating a tie is an “unnecessary piece of clothing.” (No comments about ascots, however.)
One problem with the cited study in the WSJ is that it does not link the wearing of neckties to actual infections in patients — and I don’t think any study has. Meaning this: do the patients of the necktie-wearing docs get more infections than the patients of MDs who dress more casually?
If not, then it’s just another study of this ilk: “We cultured ________ [fill in the blank of some seemingly innocuous item — computer keyboard, reflex hammer, clock radio], and found evidence of staph and coliform bacteria in XX%. These results suggest that [insert item] should be sterilized prior to patient care.”
My hunch: neckties may carry bacteria — see this company’s antimicrobial neckties for vivid proof — but they are not themselves causing nosocomial infections.
But since I could be wrong on this one, should we get rid of neckties in the hospitals and clinics just in case?
November 13th, 2009
Practical H1N1 Management Question
 Let’s imagine you’re seeing a case of pneumonia, and you suspect (as is quite reasonable these days) that it is precipitated by H1N1 influenza.
Let’s imagine you’re seeing a case of pneumonia, and you suspect (as is quite reasonable these days) that it is precipitated by H1N1 influenza.
What antibiotics do you choose for an outpatient?
(If someone is sick enough to be admitted — especially to the ICU — I’m assuming the all-guns blazing approach will be adopted.)
Even though some of these pneumonias have been only H1N1, bacterial superinfection can and does occur — most commonly with our old friend S. pneumoniae, somewhat less so with group A strep, S. aureus (including MRSA, of course), and H. influenza.
But since we hardly ever know exactly what species of bacteria we’re dealing with, how can you leave even one of these out? That MRSA one in particular?
This past week I chose trimethoprim-sulfamethoxazole + high-dose levofloxacin — in addition to the oseltamavir.
Overkill? These guidelines from Canada would suggest so, but I’m not so sure. After all, most people with H1N1 do not get pneumonia at all (and hence do not need antibiotics), and not surprisingly this was not a person with a normal immune system.
Should be an interesting winter …
November 7th, 2009
A Career in Infectious Diseases and “The Next Big Thing”
 I was working with a medical intern in clinic this past week who is potentially interested in ID. After seeing our 3rd consecutive stable HIV patient, he asked me what I thought the next big challenge would be in our field — especially since HIV treatment has been “solved.”
I was working with a medical intern in clinic this past week who is potentially interested in ID. After seeing our 3rd consecutive stable HIV patient, he asked me what I thought the next big challenge would be in our field — especially since HIV treatment has been “solved.”
“Solved” might be stating it a bit strongly — after all, we still have no cure, the drugs aren’t perfect, not everyone can get them, there’s no vaccine, etc — but he had a point. Many of the research questions on HIV treatment are now about moving things forward incrementally, and it’s hard to imagine an advance anytime soon along the lines of combination therapy in the mid 1990s, or even the second wave of newer treatments that become available in 2006-8.
So what’s the answer to his question? I compiled a brief list, shown below in no particular order:
- Highly drug-resistant bacteria — MRSA, carbapenemase-producing gram negatives, etc.
- Influenza, obviously, plus other SARS-like respiratory viruses
- Hepatitis C, though we’ll have to take this back from the hepatologists — I doubt they’ll mind — with nearly a hundred novel treatments in development
- Infections associated with therapeutic immunosuppression — TNF blockers, other biologics
- Food safety
- Device-related infections
- Novel diagnostics — PCR, other amplification techniques, direct antigen detection methods, etc
- Finding the next infectious cause of some idiopathic or autoimmune disease — some helicobacter-like discovery regarding Crohn’s, or multiple sclerosis, or sarcoid
(Not on my list are issues specifically related to ID in resource-limited settings, because that’s not what I do.)
I’m sure I’m missing something, but it’s a start.
October 31st, 2009
Would Changing Restrictive HIV Testing Laws Improve Survival?
 Emphatically yes — to the tune of >600,000* years of life gained nationwide. So says a nifty paper being presented at the annual IDSA meeting today by Mike April, under the direction of Rochelle Walensky.
Emphatically yes — to the tune of >600,000* years of life gained nationwide. So says a nifty paper being presented at the annual IDSA meeting today by Mike April, under the direction of Rochelle Walensky.
(*Original abstract said 549,437, cited in the link; number at the actual presentation, though, was 609,656.)
Bottom line is that laws that limit HIV testing (such as requiring written consent, ahem) lead to later diagnoses, and hence shorter projected life span for those with HIV. As always with such models, one could quibble with certain assumptions, but the results remain robust through a wide range of sensitivity analyses.
In fact, the only way that switching to an opt-out testing policy could fail to improve survival is if opt-out discouraged a large group of people from getting tested at all — for which there’s not the slightest bit of evidence whatsoever.
Are you listening Massachusetts? I think you are .
October 20th, 2009
Well That Was Fast! HIV Vaccine Trial Published
 Remember the HIV vaccine trial press release? The one announcing the first-ever positive result?
Remember the HIV vaccine trial press release? The one announcing the first-ever positive result?
Then the backlash, with people questioning how the analyses were done, and reported?
Now, less than a month later, we have the scientific presentation and the paper appear on the same day.
Read all about it here and here.
If you want the view from 10,000 feet (why is that the chosen altitude for that cliche?), here it is:
- The vaccine strategy combines two vaccine generally thought to be ineffective on their own — canarypox ALVAC-HIV and glycoprotein 120 AIDSVAX B/E — in a “prime and boost” approach
- Over 16,000 patients are enrolled in Thailand in a placebo-controlled trial
- The “modified intention-to-treat” analysis, which excludes those who are found to be HIV positive at entry, shows a modest but statistically significant protective effect, reducing the infection rate by about 30%
- There is a trend towards a protective effect in the intention-to-treat and per protocol analyses
- In those who were vaccinated and became infected, there was no effect on CD4 cell count or HIV RNA
Numerous questions remain, many of them summarized in this accompanying editorial: Why did it work when the individual strategies didn’t? How durable is the protection? How do the strains causing infection relate to those in the vaccine? Did the per-protocol analysis fail to show a significant protective effect solely because of a smaller sample size? Would the vaccine work if tested on higher-risk populations? What effect will this study have on the ongoing vaccine development effort, both in the lab and in trials?
Answers to some of these questions may be forthcoming. Regardless, the surprising results of this study serve as a reminder of just how mysterious the immune system remains — despite some incredibly smart people working on it with lots of resources.
Because if you asked the vaccine cognoscenti to vote a little over a year ago on which strategy in clinical trials would end up with a positive result — the “prime and boost” one published today or the adenovirus vector approach — the latter would have won in a landslide.

