An ongoing dialogue on HIV/AIDS, infectious diseases,
February 3rd, 2011
Disparities in HIV Diagnoses, and Interpreting CDC-ese
In anticipation of National Black HIV/AIDS Awareness Day (February 7), the CDC has issued a new report on the disparities in HIV diagnoses in the United States.
During 2005–2008, blacks/African Americans accounted for 13.6% of the population in the 37 states and 50.3% of the 156,812 diagnoses of HIV infection during that period … HIV transmissions in black/African American males were classified most frequently as male-to-male sexual contact (61.1%), followed by heterosexual contact (23.1%), injection drug use (IDU) (11.9%), and both male-to-male sexual contact and IDU (3.6%)… Among females, blacks/African Americans accounted for the largest percentage of diagnoses of HIV infection (65.9%) during 2005–2008. Most black/African American females diagnosed with HIV were exposed through heterosexual contact (85.2%), and the next greatest percentage by IDU (14.0%).
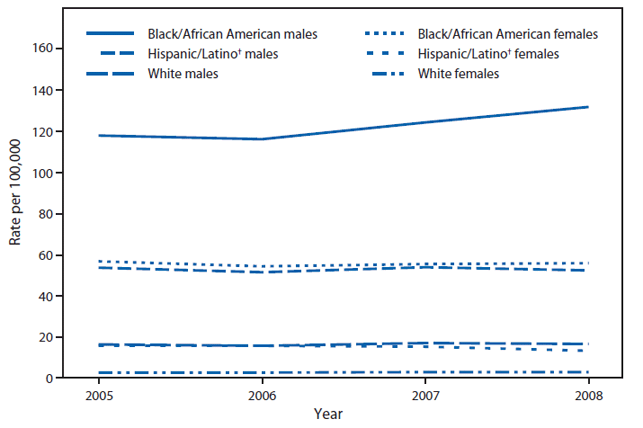
Some additional findings in this report:
- From 2005-2008, new diagnoses increased in black/African American Males; in all other groups, it was stable (see figure)
- The disparity between racial/ethnic groups was highest among persons aged 13–24 years, women, and persons with infection attributed to heterosexual contact
- In 2008, black/African American males and females were diagnosed with HIV infection at 8 and 19 times the rates for white males and females; 2 and 4 times the rates for Hispanic/Latino males and females, respectively
The authors of this report comment that “the higher rates of diagnoses among blacks/African Americans suggest that adolescents and adults from this population who are at higher risk for HIV infection might benefit [emphasis mine] from more frequent testing to facilitate earlier diagnosis.”
As usual, this is an outstanding report from the kind folks from Atlanta (who must feel this year like they’ve been tricked into living in New England).
But one of the things I’ve learned to recognize over the years of reading MMWR and other CDC reports, is that when they bring out the word “might” — a classic word from the CDC Manual of Style — what they really mean is: “We think you should do this but something is keeping us from stating it so bluntly.”
So let me be so bold: More frequent HIV testing is recommended for people at high risk for HIV — and the risk is high in certain black/African American communities, regardless of “risk factors”, as my colleague Kim Smith has wisely stated. Clinicians should be aware of the data presented here so that they can implement these recommendations appropriately.
January 28th, 2011
Wow, That Was Fast: PrEP Guidelines Appear
 With the ink barely dry on iPrEx — still tricky to type — along come “interim” guidelines from the CDC on pre-exposure prophylaxis (PrEP).
With the ink barely dry on iPrEx — still tricky to type — along come “interim” guidelines from the CDC on pre-exposure prophylaxis (PrEP).
Based on the results [iPrEx], CDC and other U.S. Public Health Service (PHS) agencies have begun to develop PHS guidelines on the use of PrEP for MSM at high risk for HIV acquisition in the United States as part of a comprehensive set of HIV prevention services. Completing the guidelines and obtaining expert input and public comment will take several months before they can be published. Concerns exist that without early guidance, various unsafe and potentially less effective PrEP-related practices could develop among health-care providers and MSM beginning to use PrEP in the coming weeks and months.
Since government organizations often move at glacial speed, the rapidity with which this appeared is truly impressive.
Some initial points worth emphasizing:
- This is just for TDF/FTC. Sure, other drugs might work — but let other studies determine that first.
- This is only for MSM at high risk for HIV. Yes, this was the group studied in iPrEx, and should be the predominant population treated. But if you knew of a heterosexual female at high risk (let’s say through domestic violence with a known HIV positive partner), would you exclude her?
- It is critical to exclude established or acute HIV infection before starting PrEP. The boxed recommendations at the bottom of the report are quite clear about this. Certainly this will increase the use of HIV RNA as a diagnostic test, and makes the combined HIV antibody/antigen assay seem much more valuable.
- Most people getting PrEP are at risk for other STIs. Screen them appropriately.
- Ongoing assessments of adherence, safety, and HIV serostatus are critical. PrEP only works if people take it, and continuing dual therapy if HIV is acquired could rapidly lead to resistance.
- Intermittent PrEP cannot be supported. Here I think the guidelines will not be widely followed, at least based on anecdotal discussions I have had with certain providers. But I understand why they needed to say this — intermittent PrEP hasn’t been proven effective — yet.
- Give a maximum of a 90 day supply. Important not to continue prescribing the drug without periodic monitoring, as noted above. I wonder where they came up with 90 days? Did they consult the rules?
I still want to know how they got this guidance out so fast — suspect they were working on it behind the scenes, awaiting publication of the study. Regardless, this is a timely release indeed, and the larger “comprehensive” set of guidelines on HIV prevention promise to be very interesting.
January 26th, 2011
Insurance Company Cheese Shop Redux
 I had an interesting exchange with one of our nurses this week about a long-term patient of ours.
I had an interesting exchange with one of our nurses this week about a long-term patient of ours.
The e-mails went something like this:
Got a fax from —-‘s insurance that his Lipitor won’t be covered anymore. They will cover simvastatin, lovastatin, and pravastatin. Let me know what you want to do.
Charlie
He’s on darunavir, and all three of those statins are contraindicated because of drug-drug interactions. Rosuvastatin?
Paul
Checked with them — rosuvastatin needs prior approval, and will cost him a lot more, but less than Lipitor. I’ll get the paperwork ready.
Charlie
An hour or so passes, and then this:
What dose rosuvastatin?
Charlie
5 mg daily, thanks.
Paul
Another hour, and then:
Just heard from them — after all the fuss, they approved the Lipitor after all. Seems they just wanted to waste our time.
Charlie
Hysterical.
Look, I get it that generics are usually more cost-effective than branded drugs. And I understand that health care costs are wildly out of control, and one way of controlling costs is to use generics whenever they are safe and effective, which is most of the time.
But think about the absurdity of the above case.
- The insurance company is paying for this man’s antiretroviral therapy, so they must know he’s on darunavir.
- They nonetheless are suggesting he switch to a contraindicated generic statin drug.
- They initially refuse to continue covering a drug that is working well and that the patient has been tolerating for years, but grudgingly will cover a slightly cheaper alternative.
- They set up barriers to jump over and tunnels to crawl through (the “prior approval” paperwork) even though there’s sound evidence to back up the requested brand-name treatments.
- After the obstacle course is navigated successfully by our experienced nurse, they relent and say that they’ll cover the original prescription after all.
And here’s the best part: The exact same thing happened last year with this patient — with the same insurance company!
Reminds me of the classic Monty Python “Cheese Shop” sketch, where the customer (John Cleese) methodically asks cheese shop guy (Michael Palin) for dozens of different cheeses — all of them unavailable. When Cleese asks at the end if they have “any cheese at all”, here’s the response:
No, sir, not a scrap. I was deliberately wasting your time, sir.
January 6th, 2011
Thursday Thienamycins
 Plenty going on in the ID, HIV, and (for the middle of winter) baseball worlds:
Plenty going on in the ID, HIV, and (for the middle of winter) baseball worlds:
- Just out in CID, there’s a comprehensive review of Management of MRSA as part of the IDSA’s Practice Guidelines Series. Soft tissue infections, bacteremia, endocarditis, pneumonia, bone and joint infections, frequent relapses … MRSA in all its painful glory. Some interesting tidbits: 1) no gentamicin recommended for MRSA native-valve endocarditis or bacteremia (a pet peeve of mine, hope that practice disappears); 2) ECHO recommended for all adult patients with bacteremia, with a preference for transesophageal ECHO; 3) no systemic antimicrobials recommended as part of “decolonization” strategies in cases of recurrent MRSA soft tissue infections; 4) no mention of household pets as a source of MRSA.
- Speaking of MRSA, ceftaroline — approved late last year by the FDA — is now actually available for use (that is, obtainable by your hospital pharmacy). This is the first beta lactam with anti-MRSA activity, and while it has been approved for community-acquired pneumonia and complicated skin and soft tissue infections, I strongly suspect it may eventually have another role — namely, management of MRSA bacteremia/endocarditis, especially refractory cases. After all, our current therapy for that condition is pretty dismal. Time (and I hope a prospective clinical trial, though nothing here) will tell whether ceftaroline in fact offers an advantage in this challenging situation.
- Etravirine now comes in a 200 mg tablet, reducing the pill burden to 1 pill twice daily. Although not approved for once-daily use, the pharmacokinetic profile supports giving the drug this way, so I wouldn’t be surprised to see a greater use of this NNRTI as a once-daily “key third drug” in patients without NRTI resistance. (It’s an “unlabeled use”, but so be it.) Turns out that although the new formulation is still uncoated, it may have reduced the quirky chalky texture of the 100 mg tablets, as tests conducted by the company show that it’s easier to swallow. It still can be dispersed in water, if patients prefer to take it this way.
- Rifaximin, the non-absorbable cousin of rifampin, reduces symptoms of irritable bowel syndrome (IBS). Great news — until you read the fine print, which shows that the placebo group did nearly as well (around a 40% response rate for rifaximin, vs 30% for placebo). Oddly, this rifaximin/IBS study comes on the heels of this well-publicized paper, which showed that placebos for IBS still worked even if patients knew they were taking them. I think we’re compelled to think again about how to leverage the extraordinary power of the mind to help control certain diseases.
- As expected (and deserved), Robbie Alomar was elected to the National Baseball Hall of Fame, receiving 90% of the votes. Does his alleged HIV positive status “pose a problem” for the Hall of Fame? I think not — so long as he does the right thing and tells the truth, whatever that may be. Again, as I’ve written before, the acceptance speech he gives in Cooperstown this summer could be the perfect opportunity.
Thienamycin’s real identity? Read all about it.
January 4th, 2011
HIV Year in Review Posted on Journal Watch
Want to catch up quickly in HIV clinical care?
Forgive the bias, but the best strategy may well be to read our “Year in Review 2010” summary over on Journal Watch: AIDS Clinical Care.
Always interesting to speculate what we’ll be choosing next year — I wouldn’t be surprised if progress in eradication (i.e., cure!) starts moving up the list …
December 22nd, 2010
Holiday Hafnias
 Some items to consider in HIV/ID world as you dig into your salmonella-free holiday bird:
Some items to consider in HIV/ID world as you dig into your salmonella-free holiday bird:
- Drug label change for stavudine (d4T): The label no longer has recommendations for dose-reduction in case of peripheral neuropathy, and cites data more strongly linking d4T use to lipoatrophy. The strategy of decreasing the dose to reduce d4T toxicity hasn’t made much sense for over a decade, since it’s long been known that most other NRTIs have less mitochondrial toxicity. Which of course begs the question — why is anyone in the United States on d4T at all?
- Speaking of HIV drug label changes, darunavir is now approved for once-daily dosing in treatment-experienced patients, provided there is no darunavir resistance. This makes sense: once-daily dosing greatly exceeds the required levels for inhibition of susceptible viruses, and the strategy was shown to be OK in the ODIN study. I suspect most of us have been doing this for many months already (certainly I have).
- Another (mostly) negative study of echinacea for colds over in the Annals of Internal Medicine. We are so desperate for something that actually works for colds that we keep flogging this dead horse. Or maybe it’s “not dead yet”? But whether this new study settles the issue once and for all, certainly any large effect of echinacea seems highly unlikely. Which reminds me — remember when pleconaril was seeking an indication for treatment of the common cold? A well-respected ID doc I know thought that if it got approved (it didn’t), hardly anyone would want it since most colds are “mild and self-limited”. But one peek at the “coughs and colds” aisle at your local pharmacy shows you just how wrong he was!
- Want the best site for the latest on the XMRV controversy? My personal favorite source is the Wall Street Journal Health blog, and here’s the most recent entry.
- For those of you vacationing in Missouri, and somehow tempted to eat raw crayfish, check out these case reports of paragonimiasis in the MMWR. My favorite sentence in the report: “Behavioral factors that led patients in this report to eat raw or undercooked crayfish included alcohol consumption, dares, and demonstration of survival skills.” Any surprise that 8 of 9 of the cases were in males?
Happy Hafnias!
December 17th, 2010
Update on Berlin Patient II: Still Cured of HIV
First, who was Berlin Patient I?
Second, over in the journal Blood is the latest update on Berlin Patient II, the guy apparently cured of HIV by bone marrow transplantation:
We have previously reported the case of an HIV-infected patient in whom viral replication remained absent despite discontinuation of antiretroviral therapy after transplantation with CCR5Δ32/Δ32 stem cells… In the present study, we demonstrate successful reconstitution of CD4+ T cells at the systemic level as well as in the gut mucosal immune system following CCR5Δ32/Δ32 stem cell transplantation, while the patient remains without any sign of HIV infection… In conclusion, our results strongly suggest that cure of HIV has been achieved in this patient.
Ever since this was first presented as a poster at CROI in 2008, it was pretty clear that this was a special case, and I’ve written about it several times before — first time here.
What’s different now, of course, is that the longer duration of follow-up has allowed the authors to give much more detailed information about both his virologic and immunologic status — and to make more confident statements that he is in fact cured of HIV.
 And I agree.
And I agree.
But numerous questions about this fascinating case remain, including the biggie — how did this happen? Was it the CCR5-negative status of the donor’s cells? Something about the “conditioning” (there’s a euphemism for you) regimen? The fact that he’d been virologically suppressed when he went into the transplant? (It’s obviously not just this.) Some combination of the above?
And though of course this transplant strategy can’t be widely adopted, one would expect at least one other similar case to surface soon, right?
By the way, for HIV history trivia buffs, this guy was Berlin Patient I. Must be something in the water.


 As another major snow storm
As another major snow storm  January 1, 2011
January 1, 2011