An ongoing dialogue on HIV/AIDS, infectious diseases,
April 9th, 2011
And Now, for a More Comprehensive CROI Report …
 Although I’ve already provided a Really Rapid Review™ of the 18th Conference on Retroviruses and Opportunistic Infections (CROI), the editors of Journal Watch/AIDS Clinical Care have put together a more comprehensive summary here.
Although I’ve already provided a Really Rapid Review™ of the 18th Conference on Retroviruses and Opportunistic Infections (CROI), the editors of Journal Watch/AIDS Clinical Care have put together a more comprehensive summary here.
I sometimes wonder what research from these conferences will not only stand the test of time, but will grow in importance and be viewed one day as a landmark event.
Past notable examples include the first triple-therapy study with indinavir — which showed that virologic suppression could be durable — presented way back in 1996 at the 3rd CROI, and the phase III study of raltegravir in patients with highly drug-resistant virus, presented 11 years later at CROI 14.
My bet this time? While it’s notoriously tough to make predictions, especially about the future, my vote goes to these zinc finger nuclease studies — which are part of a very concerted and growing effort to cure HIV.
April 5th, 2011
Scamming Academic Journals
Academic scams are not just limited to meetings.
Every so often, I receive an e-mail that goes something like this:
Dear Dr. Sax,
The journal Contemporary Organic Biosynthesis [journal name made up] covers all the latest and outstanding developments in organic biosynthesis studies. It is one of the leading journals for expert reviews in the field. Please visit the journal’s Web site at www.organicbiosynthesisreviews.com to see our Mission and Scope.
It is my pleasure to invite you to submit the title of your article, a one-page abstract and the submission deadline for the Editor-in-Chief’s approval to editorial@obsr.com. We also invite you to to consider to guest edit a thematic issue to the journal of 5 – 12 invited articles from colleagues of your choice.
Guest Editors of thematic issues and all main contributing authors will receive a free online subscription to the journal for one year.
Sincerely,
Griffila Zaporsky-Branson, PhD [I also made that name up]
Editor-in-Chief
As you probably know if you’re reading this site, I am barely more qualified to write a review on “organic biosynthesis” than Oprah Winfrey. So obviously this is a form letter sent to hundreds (if not thousands) of academic MDs.
But beyond that, how do “journals” like this make money? Is there a large author fee once the scholarly review is written and “approved” by Dr. Zaporsky-Branson? Do they sell advertisements? Do they require a valid credit card before the requested reprints are sent out, with money neatly diverted to someone’s bank account?
Regardless, as with the Nigerian e-mail scams, obviously someone is biting — otherwise these emails wouldn’t exist at all.
April 1st, 2011
Clindamycin or Cephalexin for (Mostly) MRSA?
 Over on the Journal Watch Pediatrics site, there’s a summary of a study that compared clindamycin with cephalexin for purulent skin infections in kids age 6 months to 18 years. The results?
Over on the Journal Watch Pediatrics site, there’s a summary of a study that compared clindamycin with cephalexin for purulent skin infections in kids age 6 months to 18 years. The results?
MRSA and methicillin-susceptible Staphylococcus aureus (MSSA) were isolated from 70% and 19% of children, respectively … The primary outcome — clinical improvement at 48 to 72 hours — was similar in the cephalexin and clindamycin groups (94% and 97%, respectively), as was the secondary outcome — resolution of infection at 7 days (97% and 94%).
Should we be surprised that cephalexin — an antibiotic with notoriously poor systemic absorption — did so well vs. infections that were 70% resistant to the antibiotic?
Not really — after all, if antibiotics were required for skin and soft tissue infections, we would have perished as a species in the many thousands of years before the discovery of penicillin. Most of the cure comes from the local care, which was done in 97% of patients.
Plus, this study of cephalexin vs placebo in adults found the same exact thing.
Finally, it proves something that Primary Care Providers, Emergency Room Doctors, Surgeons, and Pediatricians have known for some time: If you want to use a weak antibiotic (i.e., almost a placebo) “just in case,” hardly anything beats our old friend “Keflex.”
March 26th, 2011
Zoster Vaccine for Age 50 and Up? A Resounding “Yea” Vote Here
 I was getting off the elevator at the hospital the other day, and a cardiologist greeted me with the phrase every ID doctor in the world will instantly recognize:
I was getting off the elevator at the hospital the other day, and a cardiologist greeted me with the phrase every ID doctor in the world will instantly recognize:
Can I ask you a quick question?
It was actually a series of questions, and, as is often the case, it wasn’t so “quick”. But I was happy to help.
Her sister — age 57, living in Virginia — had just been diagnosed with ophthalmic zoster, and was having a very rough time of it. Lots of pain, swelling, and of even greater concern, corneal involvement with markedly reduced vision.
The problem, of course, is that there’s only so much that antiviral therapy can do once shingles is diagnosed. Far better, of course, is to prevent it in the first place.
Then the next day the FDA approved the zoster vaccine for people aged 50-59, reducing the age threshold by 10 years.
The reasons for approval are plain enough. Around 200,000 people this age get shingles each year in the United States, and the vaccine reduces the risk by 70%. So it’s even more effective in this young patient population (and I chose that adjective intentionally, ahem) than in those for whom it was originally approved.
Let’s hope the distribution and cost issues — which are substantial — are resolved soon, as this is one adult vaccine I most heartily endorse.
March 18th, 2011
Friday Fosfomycins
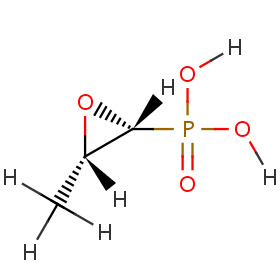 Today’s ID/HIV comments and links are named after every ID specialist’s favorite new toy for UTIs.
Today’s ID/HIV comments and links are named after every ID specialist’s favorite new toy for UTIs.
- This HIV transmission from a kidney donor is getting quite a bit of media play, as such complications always do. I was at a meeting this AM when one of my colleagues (an endocrinologist) commented how horrible she thought it was. Yes… but here’s what I told her: the risk of some sort of infectious complication from transplantation, despite all our vigilance, no matter what tests we use, will never be zero. The only way to make transplants 100% safe is to stop doing them entirely. And that’s not going to happen, nor should it.
- Someone told me that CROI 2012 will be in Seattle “in late February.” CROI trivia hounds will recall it was in Seattle in 2002, when we all learned the MTD (maximal tolerated dose) of caffeine. No confirmation on the date (naturally) from the CROI web site, so I wouldn’t book your flights just yet.
- Those of you who do inpatient ID consults will recognize some of the absurd dynamics in play in this animated (literally) conversation. It’s an extreme example, but I confess I laughed out loud a few times anyway (sorry).
- Speaking of inpatient ID consults, I’m on service right now, and of course it’s staph, staph, and more staph. (First-year ID fellows sometimes think they’re doing a Staph aureus fellowship.) All this staph means I’ve had the opportunity to get some anecdotal experience with ceftaroline for MRSA. Since we learn in Statistics 101 that there’s nothing less scientific than anecdotal (especially early anecdotal) evidence, I’ll resist the impulse and see how things go for a bit longer, and will only say that the drug is tricky for home IV administration since it needs to be mixed up each day. Any other impressions out there?
- Finally, for a pretty pessimistic view of the “test and treat” HIV strategy, here’s a discouraging review of how few of the people living with HIV are actually engaged in care. By the estimates presented here, just under 20% of HIV-infected individuals in the United States have an undetectable HIV viral load. If the data are right, this figure says it all — and regardless of whether there are 209,773 or 209,774 out of 1,106,400 with suppressed HIV, there’s plenty of room for improvement.
Hat tip to the inimitable Rochelle Walensky for the video!
March 8th, 2011
Really Rapid Review of CROI 2011 — and No CROI 2012 Dates
 With CROI 2011 now officially over, I offer below the following Really Rapid Review™ for ID/HIV Specialists with limited time — or for those who said they went to the conference but spent the entire week shopping in the Prudential Mall and eating at Legal Seafood:
With CROI 2011 now officially over, I offer below the following Really Rapid Review™ for ID/HIV Specialists with limited time — or for those who said they went to the conference but spent the entire week shopping in the Prudential Mall and eating at Legal Seafood:
- Lots on PrEP. Bottom line — it works if you take it, but lots of people in iPrEx didn’t take it, especially in non-US sites. And bone density goes down a bit in those receiving TDF/FTC, long-term implications not known. Based on conversations I had with colleagues, it didn’t seem that anyone had been prescribing PrEP much thus far.
- HCV treatment is about to get much more effective, and much more complicated. The drug-drug interactions with antiretroviral drugs and the first two HCV-protease inhibitors — telaprevir and boceprevir — are going to be really, really dicey. Sensational web-cast here of a plenary given by Stefan Zeuzem that summarizes lots of the key issues.
- Once-daily raltegravir doesn’t work quite as well as twice-daily. Old news, but detailed data on the trial presented here for the first time. Turns out PK does matter after all in the QD group.
- Speaking of raltegravir, if you predicted that approximately 25% of treatment-naive subjects who were put on boosted darunavir plus raltegravir would experience virologic failure, you are smarted than I am. These are two of our best drugs — how can we explain this?
- Time to learn a new drug name: “S/GSK1349572” = “572” = “dolutegravir” = “DTG”, which really does have antiviral activity against some raltegravir-resistant viruses.
- Two additional studies (here and here) show that blacks in the USA do worse than whites in clinical trials. The explanation must be at least in part socioeconomic, since clearly Africans in Africa are doing just as well on ART as people in resource-rich settings. Critical to figure out why we have this disparity.
- Many studies on inflammation and immune activation, including this incredibly counter-intuitive study of maraviroc intensification of already suppressive ART. Results are too complex for this Really Rapid Review™, but they go something like this: certain markers increased; others decreased; no one knows why; no one knows the clinical implications. Which pretty much sums up this whole “inflammation and immune activation” field right now.
- If you have active TB and advanced HIV-related immunosuppression — that is, CD4 < 50 — the time to start ART is sooner rather than later. (Similar findings in this study.) All this makes complete sense, given what we’ve seen from earlier studies in such sick patients (A5164, CAMELIA). Fact: Untreated advanced AIDS is worse than IRIS.
- Several years ago, plunked right into the middle of a slide session on clinical studies of antiretrovirals, came an out-of-nowhere basic science presentation (this might have been it) on “zinc fingers”. So odd was its appearance that it became something of an inside joke (a very inside joke) among certain HIV clinicians. (“Oh, he can’t tolerate ritonavir — maybe he’d do better on a zinc finger inhibitor.”) And guess what — zinc fingers are back! And it’s actually very cool stuff.
- Does PI-based ART in the mom lead to premature delivery? The Europeans have been saying so for some time, and this randomized clinical trial from Botswana supports that view. The questions remain — how clinically important? And what are the best alternatives?
- You know that question about whether NRTIs are needed in 2nd-line therapy (and beyond)? Based on these two studies (here and here), it certainly seems so. A randomized clinical trial — the so-called OPTIONS study — is ongoing to try and answer this question definitively.
Finally, unlike last year, if the CROI 2012 dates were announced, I didn’t hear them — nor did anyone else I spoke to at the conference. Darn. That “announcement” up there is a mock-up kindly crafted by my sister, who always did help me with my art projects in grade school.
But in case the conference organizers are still looking, there’s plenty of space at the Hynes Convention Center should they want to come back to Boston. Right now, this group is the only meeting booked for the whole month of February!
February 27th, 2011
CROI 2011 Starts Today
 With a fresh 4 inches of snow on the ground in Boston, the 18th Conference on Retroviruses and Opportunistic Infections (CROI) starts today.
With a fresh 4 inches of snow on the ground in Boston, the 18th Conference on Retroviruses and Opportunistic Infections (CROI) starts today.
Pocket program is available here (PDF format). Based on a (very) quick perusal, we can expect the following:
- Progress (lots of it) in prevention, with more from CAPRISA and iPrEx and the whole “treatment as prevention” theme
- Welcome HIV specialists to the new age of HCV therapeutics
- Several big clinical trials, including more details on once-daily raltegravir and studies on early vs deferred ART for HIV-related TB
- Numerous posters and presentations on cardiovascular, renal, neoplastic and bone complications
- Diverse studies on the maturing treatment programs in resource-limited settings
As always with CROI — which is the most concentrated (in a good way) of our scientific meetings — there will be a ton of miscellaneous other interesting stuff.
Come back here later for a more detailed summary and (if they announce it) the date of the 2012 conference!
February 17th, 2011
Zinc May Work for Colds — But Don’t Pretend It’s Not a Drug
Does zinc work for colds?
 Apparently it does, according to this Cochrane Review. From the “Plain Language Summary”:
Apparently it does, according to this Cochrane Review. From the “Plain Language Summary”:
This review identified 15 randomized controlled trials, enrolling 1360 participants of all age groups, comparing zinc with placebo (no zinc). We found that zinc (lozenges or syrup) is beneficial in reducing the duration and severity of the common cold in healthy people, when taken within 24 hours of onset of symptoms. People taking zinc are also less likely to have persistence of their cold symptoms beyond seven days of treatment.
Based on these results, and the ecstatic “I-told-you-so” reader comments on this New York Times report, there is likely something real going on here. It’s potentially pretty exciting, actually — a treatment for the cold!
But one thing is clear: Zinc for colds should be considered a drug — even though you can get it without a prescription, the FDA hasn’t reviewed it, and the lozenges may carry the word “natural” or “organic” on the label.
Why? Because in addition to their benefits, all drugs have side effects. And because there’s nothing “natural” about zinc supplements in people who don’t have zinc deficiency, it won’t be long before more side effects (remember this one with zinc nasal swabs?) or drug interactions occur in a least some individuals.
So stay tuned for further research on this area — and be ready for results that may be good and not-so-good.



