An ongoing dialogue on HIV/AIDS, infectious diseases,
September 17th, 2011
Drinking Coffee Prevents MRSA
 I follow the medical literature on coffee very closely.
I follow the medical literature on coffee very closely.
Why? Because I’m completely addicted — and, judging from the lines at the Starbucks, Dunkin’ Donuts, etc at the airports before early morning flights, I am not alone.
(It’s just one cup a day. Any more and say hello to palpitations, jitters, sweats, and long sleepless nights. Is there ever such a thing as a short sleepless night?)
So I thank a former ID fellow for pointing out this key paper:
We performed a secondary analysis of data from the 2003–2004 National Health and Nutrition Examination Survey to investigate the relationship between the consumption of coffee, hot tea, cold tea, and soft drinks, and MRSA nasal carriage …Individuals who reported consuming coffee had about a one-half reduction in the risk of MRSA nasal carriage relative to individuals who drank no coffee (odds ratio = 0.47; 95% confidence interval, 0.24–0.93).
(Brits will take solace that drinking tea was similarly effective.)
And let the record show that despite various researchers trying to blame coffee for ulcers, high blood pressure, coronary artery disease, gout, birth defects, anxiety, and several cancers — most notoriously pancreatic cancer — the evidence that it causes any of these things is weak at best. In fact, the coffee/pancreatic cancer paper is taught in some statistics classes as an example of how poorly designed case-control studies can give misleading results.
So in defense of this one cup a day addiction, let’s bring on more articles on the health benefits of coffee.
September 11th, 2011
Must-Read Paper: “Antiscience” and Lyme Disease
As I’ve written before, there are few clinical encounters more challenging for Infectious Diseases specialists than the patient who, despite negative standard diagnostic testing, believes he/she has Lyme disease.
Now, in Lancet Infectious Diseases, comes a paper entitled “Antiscience and ethical concerns associated with advocacy of Lyme disease.” It meticulously describes the distinctive world of alternative diagnosis, treatment, and passionate advocacy related to Lyme.
Some highlights — first, the background:
As with other antiscience groups, some Lyme disease activists have created a parallel universe of pseudoscientific practitioners, research, publications, and meetings, arranged public protests and made accusations of corruption and conspiracy, used harassment and occasional death threats, and advocated legislative efforts to subvert evidence-based medicine and peer-reviewed science.
And those odd diagnostic tests, often paid for out-of-pocket by the patient?
Despite warnings from the US Food and Drug Administration and the CDC about the potential unreliability of unvalidated diagnostic tests for Lyme disease, many LLMDs [Lyme-literate MDs] continue to use such assays. [Specific assays are cited in the full article.] Lyme specialty laboratories are favoured by some activists and LLMDs because their non- standard testing methods and interpretation criteria often lead to more positive results than other laboratories that rely on validated methods.
What to do?
Many patients who have been labelled as having chronic Lyme disease arrive at this diagnosis as a consequence of inadequate or frustrating previous medical care for symptoms that are difficult to define. Patients who suspect or who have been diagnosed with chronic Lyme disease should consider seeking a comprehensive assessment from an empathetic physician..
My advice: Read the full article. (That’s the polite form of RTFA.)
September 4th, 2011
“Novel” Approaches to Initial HIV Therapy: Part II
 Two studies were just published on alternative strategies for initial HIV therapy. I’ve already reviewed the first one here.
Two studies were just published on alternative strategies for initial HIV therapy. I’ve already reviewed the first one here.
The second paper is a single-arm (n=112) study of darunavir/r (once daily) plus raltegravir, the latest riff on the “NRTI sparing” approach.
As I mentioned when I first covered this study, the high rate of virologic failure — 26% overall, and a whopping 43% (21 of 49) with viral loads > 100,000 — came as a complete shock. In fact, I would have bet good money that this regimen would work just fine. (Glad I’m not a betting man — except in poker.) Five of the 28 virologic failures developed integrase resistance, all of them from the high VL stratum.
Why is this so surprising? You have the antiviral potency of raltegravir plus the high genetic resistance barrier of boosted darunavir — so what went wrong?
The study authors offer some possible explanations for these disappointing results (suboptimal adherence, asymmetrical dosing, lowered darunavir concentrations from raltegravir, minority variants resistant to raltegravir), but the bottom line is that this regimen just didn’t work very well. A fully-powered comparative study (darunavir/r plus raltegravir or TDF/FTC) is ongoing in Europe; I’m sure their DSMB is keeping a close eye on the results.
So two non-standard approaches to initial HIV therapy, with TDF/FTC + etravirine looking promising, and darunavir/r + raltegravir much less so.
September 3rd, 2011
“Novel” Approaches to Initial HIV Therapy: Part I
It’s been several years since the “preferred” or “recommended” initial regimens for HIV treatment have been consolidated into one of the following four:
- TDF/FTC + efavirenz
- TDF/FTC + atazanavir/r
- TDF/FTC + darunavir/r
- TDF/FTC + raltegravir
Any room for improvement in this “TDF/FTC + key third drug” approach? With the recent approval of TDF/FTC/rilpivirine, certainly this will have a role in some patients, but the issues of excess virologic failure and resistance in those with high viral loads will likely limit its use.
And though extended release nevirapine has been available for months, I confess it hasn’t really occurred to me to use it in a patient starting treatment given the other options out there. (And even those who are currently stable on NVP twice daily don’t seem eager to switch — I’ve offered.)
Now along come two interesting papers testing alternative initial treatment strategies. Both have quite interesting results that definitely got my attention when presented earlier this year at major meetings, small size of the studies notwithstanding.
The first is a blinded, randomized trial comparing etravirine and efavirenz, both given with 2 NRTIs. Note that the etravirine was dosed once-daily — not the twice-daily manner in which it is approved — and given in the older 100 mg (very crumbly) tablet formulation. Note also that the primary endpoint was the percentage of patients with Grade 1-4 drug-related CNS side effects, and not virologic efficacy. Since it was powered off differences in this side effect, only 157 patients were enrolled, not the 600 or so typically seen in a comparative study of efficacy.
Nonetheless, the results are impressive: 76% vs 74% in the etravirine and efavirenz arms respectively have VL < 50 at week 48, and for the group with baseline VL > 100,000, the results are 74% vs 67% — which is perhaps the first time in HIV study history that a regimen is numerically superior to EFV in this high viral load stratum. Also of interest, among the four patients with virologic failure on etravirine, zero show emergent resistance mutations — very unusual for an NNRTI — vs 3 of 7 for efavirenz.
Oh, and the primary endpoint — the occurrence of CNS side effects — significantly favors etravirine, as do lipid changes.
These results have to be considered encouraging on multiple fronts, including efficacy (caveat: small sample size, I know), resistance, tolerability, and lipid effects. Plus, with the newer 200 mg formulation of etravirine, this is an initial regimen of just three pills once-daily. Consider me impressed.
(Nerdy HIV specialist historical quiz question: how many pills/day was etravirine when in phase I/II studies? Hint, it was a lot.)
For another novel approach to initial therapy, read here.
August 31st, 2011
It’s Time for Antibiotic Placebos
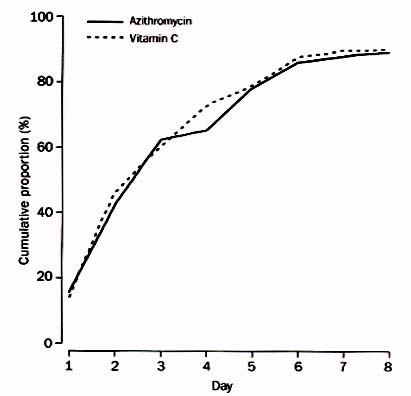 As I’m sure you all agree, it’s high time we had a good antibiotic placebo.
As I’m sure you all agree, it’s high time we had a good antibiotic placebo.
Just think — we’d be able to prescribe a 100% effective treatment for viral respiratory tract infections, with the assurance of no risk of antibiotic resistance, C diff, allergic reactions, tendon ruptures, photosensitivity, drug-drug interactions, or any of the myriad other side effects that real antibiotics have.
Plus, based on studies like this one, we could even tell our patients that they’re receiving an antibiotic placebo, and they’d still get better. There, ethical quandaries of placebo prescribing solved.
What inspired this thought on antibiotic placebos was this email exchange:
Hi Paul,
Our primary care group would love to have you come give an update on antibiotics during our Friday conference. Am cc’ing Chuck our practice director to make sure he agrees with the topic. There’s a lot of variability in antibiotic prescribing within our group, hoping you can enlighten us.
Thanks,
Nora
So Chuck chimed in:
Sounds great. But Nora, what do you mean “variability in antibiotic prescribing” if everyone gets azithro?
Chuck
Now it hardly bears mentioning that Chuck is one of the funniest doctors I know, and that he was being facetious.
But just think how much macrolide resistance we could avoid if, instead of a “Z-pack”, we had an “S-pack” (“S” for sucrose). That’s 6 pills (two on the first day, then one a day for 4 additional days), with the promise that it will be just as effective as azithromycin.
And if you don’t believe me, read this paper in The Lancet for proof (figure from paper in above image). And this news article in The Onion for more information.
Sucramycin (brand name Sucracil®), anyone?
August 24th, 2011
Hepatitis C and … Baseball?
 From the prolific folks at NATAP came this surprising announcement:
From the prolific folks at NATAP came this surprising announcement:
The Cardinals are stepping up to the plate against Hepatitis C. Starting Monday, August 22, fans will be able to get free Hepatitis C screenings at Busch Stadium. It’s part of a nationwide effort by Major League Baseball to bring attention to the causes and treatment of Hepatitis C.
Cardinal fans — and believe me, there are lots of them out there — will note that this opportunity comes the same month as the Willy McGee and Matt Holliday Bobblehead games, coming up this Thursday and Friday respectively.
But giveaways aside, why is MLB doing this? There are lots of diseases out there for which they could offer screening, though clearly colon cancer would be hard to pull off in a ballpark.
One possibility is that the teams that are offering it — the Cardinals, Astros, White Sox, and (of course) Cubs — need a distraction from the fact that the odds of their appearing in the post-season are fading daily.
Another is that HCV is still looking for it’s ideal Magic Johnson-like spokesperson, something John Bartlett (the Hopkins one) has always said the disease lacks. Plenty of bad candidates over the years — Evel Knieval, Jack Kevorkian, James Earl Ray, Linda Lovelace.
So is some appealing baseball superstar about to step forward?
Let’s just hope he’s not on the Brewers — beer and HCV not a great mix.
August 19th, 2011
A Reason To Continue Restrictive HIV Testing Laws? Not Really …
 The pending HIV legislation is much on my mind these days, for reasons I outlined here. Bottom line is that I don’t think it’s good for patient care, and we’re missing a real opportunity to make things better here in the Bay State.
The pending HIV legislation is much on my mind these days, for reasons I outlined here. Bottom line is that I don’t think it’s good for patient care, and we’re missing a real opportunity to make things better here in the Bay State.
But yesterday I heard a perspective on the bill I hadn’t considered, and it went something like this:
We still need the laws about HIV testing — and the added protection of privacy — because of the disgraceful response of the medical community to AIDS when it first burst on the scene in the 1980s.
Examples: The physician who refused to operate on an AIDS patient. The hospitals that tried to avoid having AIDS cases since it would scare other patients away. The thoughtless release of the AIDS diagnosis to employers, family members, friends. And so on.
And I get that — I understand that doctors, nurses, policy makers, and the rest of the large community that makes up the “medical system” didn’t always behave so wonderfully when faced with this new and scary disease. Back in 1989, I heard a director of a Cardiac Intensive Care unit say that having an AIDS patient in his unit was a “waste of resources.” That wasn’t right then, and it isn’t right now.
But here’s some items to consider as a counter argument:
- It was people from all aspects of society behaving badly, not just the medical community. Remember Ryan White? In fact, I’d argue it was a relatively smaller proportion of health professionals compared to the rest of the population.
- Things are different now — so very different. HIV is treatable, for everyone who can get diagnosed and into care. Check out this paper from Hopkins, just published, outlining just how treatable it is, even in a mostly inner city, poor, minority population. Not meaning to diminish the seriousness of HIV in any way, I would argue that this single fact — the treatability (is that a word?) of HIV — makes restrictive HIV testing laws obsolete. Paul Farmer has said repeatedly that making a fatal disease treatable dramatically reduces it’s stigma. And who are we to disagree with Paul Farmer?
- Didn’t the medical community also have the opposite response? The doctors, nurses, social workers, research scientists, and other health professionals who devoted their combined efforts to caring for people with HIV, and improving their prognosis? Seems that this should also be acknowledged when citing painful anecdotes about bad behavior.
And since these dedicated HIV specialists are universally in favor of removing restrictions on HIV testing and the proposed barriers to provider communication, that must be telling us something.
August 11th, 2011
Next Single-Pill HIV Treatment Approved, and It’s Not Called “B-Tripla”
 One famous HIV clinician/clinical researcher likens co-formulated TDF/FTC/EFV (Atripla) to a “Godzilla,” so dominant has the treatment become as initial therapy for HIV. He bases his comments on this study done at his institution, showing that in 2007, fully 85% of patients starting treatment in their clinic began TDF/FTC/EFV.
One famous HIV clinician/clinical researcher likens co-formulated TDF/FTC/EFV (Atripla) to a “Godzilla,” so dominant has the treatment become as initial therapy for HIV. He bases his comments on this study done at his institution, showing that in 2007, fully 85% of patients starting treatment in their clinic began TDF/FTC/EFV.
Does this big lizard of a regimen now have a competitor? Maybe:
On August 10, 2011, FDA approved Complera, a fixed dose combination (FDC) drug product containing emtricitabine/rilpivirine/tenofovir DF (FTC/RPV/TDF) for the treatment of HIV. The recommended dose of Complera™ is one tablet, containing 200mg/25mg/300mg of FTC/RPV/TDF, once daily, taken orally with a meal.
A couple of quick thoughts as I get used to the name:
- As I’ve written before, the data suggest that using TDF/FTC/RPV involves a trade-off between safety/tolerability (favoring RPV) and efficacy (favoring EFV). Will this efficacy difference be reduced now that the single-pill treatment is available? This study is testing that very question. For now, probably best to limit use to those with HIV viral load < 100,000.
- Are people going to be using TDF/FTC/RPV as a switch strategy from virologically suppressed patients? The answer to that is undoubtedly yes, though of course this will be an off-label use, and clinicians might get push-back from payors. (I did already when trying to prescribe TDF/FTC + RPV separately to a treatment-experienced patient.) Again, ongoing studies are testing this switch strategy, both from boosted PI-based regimens and from EFV — the latter particularly important since EFV induces metabolism of RPV, lowering levels for a least a few weeks.
- Rilpivirine must be taken with a meal — not just food, but a meal — and it’s not clear yet whether this will be something that all patients can reliably do in clinical practice.
- One area where I’m sure the treatment will get some traction is in women of childbearing potential, as some clinicians are leery of prescribing EFV. One caveat of course is that there are no actual pregnancy data yet on this new drug, but at least at the outset RPV is a Category B drug (EFV is category D).
- We should expect the usual delay between this approval and the appearance of the regimen on ADAP, other government, and private insurance formularies.
Isn’t it odd that pronunciation of these new drugs is often a mystery? I’m still not sure whether it’s etra-VIR-ine or e-TRA-virine, IN-telence or In-TEL-ence, and is “Edurant” pronounced EE-durant or EH-durant or ee-DUR-ant?
As for Complera, I’ll assume the accent is on the second syllable until I hear otherwise.
August 3rd, 2011
Why the Proposed Massachusetts HIV Testing Bill is Bad for Patients
 As I’ve written about here multiple times, I’m not a big fan of the HIV testing law in our state.
As I’ve written about here multiple times, I’m not a big fan of the HIV testing law in our state.
First, there’s the requirement for written informed consent, something that every state (except a couple) has wisely abandoned. Second, it’s more than a testing law — it’s also an HIV privacy law, which is arguably unnecessary in this post HIPAA era and has all sorts of unintended consequences.
Last year, two bills were proposed — one simply removed the requirement for written consent, while the other replaced it with a requirement for verbal consent. The groups backing these respective bills (I was part of the former) didn’t work out a compromise, and so no bill was passed.
This year? Something very strange has happened. We have a bill pending “An Act to Increase routine screening for HIV” that would remove written consent but would also expand HIV-related privacy protections and mandate that primary care and ID providers “offer” HIV tests to their patients. The bill is working its way through government channels, on its way to being passed.
And you know what? I’m not aware of a single ID or HIV clinician who supports it.
This includes providers at hospitals, community-based clinics, group practices, and the largest medical practice in the state for gay and lesbian people. That’s right — most of the people dedicating their careers to HIV care don’t like it.
Here’s why this bill is a problem:
- It requires documentation that verbal consent for HIV testing was obtained. I’ve been told that in NY, where a similar law exists, many institutions haven’t removed the requirement for written consent because of this provision — patients still have to sign a form. In short, practice hasn’t changed even though the law has.
- The definition of protected “HIV-related information” has been broadly expanded, with a requirement for written patient consent before this information is shared. As currently written, the proposed law would block clinician-to-clinician referrals if they are not in the same facility, or a simple release of information from a hospital to a community PCP — discharge summaries, test results, diagnoses, medications. All would require written consent from the patient first. (Note that this goes well beyond the existing requirement for written consent to release medical records — this we already do for everyone, not just those with HIV.) Needless to say, no other disease has this restriction, and to say this is an obstacle for good patient care is a huge understatement. How would this even be applied to electronic medical records, which are increasingly being used in the community?
- There is language mandating that primary care and ID clinicians must offer an HIV test to a patient unless it is documented that the test has been done before. Is there another screening test (colonoscopy, mammography, TB skin testing, hepatitis C) where a law exists that clinicians must offer the test? Does this increase a clinician’s legal risks around HIV testing? And how do we access whether the test has been done if it’s protected information, as noted in #2? Recommendations for HIV testing should fall under the province of clinical guidelines (such as those issued by CDC), not under law.
- There is no way to test critically ill patients who are unable to give consent unless they have a health care proxy to consent for them. Anecdote: I saw a 35-year-old woman with a large brain abscess late last year who was comatose. An HIV test would have been invaluable to help distinguish bacterial brain abscess from possible toxoplasmosis — conditions which obviously require different therapies — but she couldn’t give consent and, as is very common in younger patients, she had no health care proxy. Wouldn’t it make more sense to allow HIV testing in instances like this, where the treating clinician could provide better care if he/she knew the patient’s HIV status? This is obviously how testing for other conditions in critically ill patients is done. Notably, the argument to allow testing for HIV in this setting was brilliantly made by a bioethicist writing way back in 2005.
- There is no way to allow testing of source patients in occupational exposures unless the source gives consent. In some states, antibody testing on previously-obtained blood samples is permitted. I’ve expressed this view before, but in occupational exposures, the benefits of knowing a source patient’s HIV status extend to both the source patient and the exposed health-care worker. Shouldn’t we do everything we can to make obtaining this information as easy as possible?
I know the proposers of the bill have the best interests of people living with HIV in mind, but the problems listed above suggest that clinicians were not part of the development process. Since the bill has been on the table, several HIV clinicians and researchers (including me) met with the sponsor to discuss our concerns, but to no avail. For the record, the bill is also opposed by the Massachusetts Medical Society, who articulated these concerns at a public hearing earlier this year.
So let’s change the law, yes. But let’s get it right please:
- Remove the requirement for written consent.
- Allow existing privacy protection statutes (which did not exist in 1986, when the current HIV testing law was enacted) to apply to HIV.
If you live in Massachusetts and care about this, find your Senator and Representative, and let them know that this bill is bad for patients — and has the unintended effect of being discriminatory, leading to inferior care for people living with HIV.
You can bet I’ll do the same.
July 28th, 2011
Really Rapid Review — IAS 2011 Rome
 Just back from IAS 2011 (which was followed, I’m thrilled to say, with a visit to perhaps the most beautiful region in the world). Here is a Really Rapid Review™ of the meeting, with apologies ahead of time for lack of organization and (even more likely) leaving out something important. FYI, the abstracts are online here; I’m sure there’s a place on the meeting homepage that has the same link, but I can’t seem to find it.
Just back from IAS 2011 (which was followed, I’m thrilled to say, with a visit to perhaps the most beautiful region in the world). Here is a Really Rapid Review™ of the meeting, with apologies ahead of time for lack of organization and (even more likely) leaving out something important. FYI, the abstracts are online here; I’m sure there’s a place on the meeting homepage that has the same link, but I can’t seem to find it.
- All 052, all the time. This landmark study was the big story at the conference, with several presentations on it (and the paper published the same day in the NEJM; Journal Watch comment here). Very interesting fact: the only linked transmission in a person whose partner was randomized to early therapy probably took place before virologic suppression. At the end of the series of talks on the study — which covered the primary outcome, details on how they documented viral linkage, and the clinical, immunologic, and virologic results — the investigators (led by Mike Cohen) received a standing ovation from the audience. Well deserved. (Quick aside: Did they ever tell the participants whether the HIV transmission was linked to the infected partner? I suspect not, but …)
- What about PrEP? Results from two favorable studies (abstract here for one, slides here for the other) were released just before the conference, and further details were presented right after the 052 data. Important studies? Yes. “Game changers” for clinical practice, like 052? Probably not.
- Dolutegravir looks really, really good. Week 48 data from a relatively large phase II naive study show around a 90% suppression rate, no obvious toxicity, and — amazingly — no integrase-inhibitor resistance among virologic failures. Could dolutegravir be like a boosted PI in that regard? This smart guy thinks so. In an odd coincidence of recent HIV drug development, dolutegravir seems to do the same thing with inhibition of creatinine secretion (thereby raising serum creatinine without reducing GFR) as cobicistat.
- Speaking of integrase inhibitors, once-daily elvitegravir is “non-inferior” to raltegravir in treatment-experienced patients. Good news, as the phase II study of elvitegravir was highly problematic, in hindsight due to both dosing issues and the absence of active background therapy. Note that in this phase III study, elvitegravir was given with a PI boosted by ritonavir, not by cobicistat. And cobicistat sounds great with an Italian accent.
- Any other promising new investigational drugs out there? Maybe –The NNRTI lersivirine was almost as good as efavirenz in this phase II study. Since phase II studies tend to be relatively small, any signals of inferior efficacy are necessarily a concern.
- In a similar vein, the two-drug regimen of ATV/r and QD maraviroc was (again) almost as good as ATV/r + TDF/FTC. Specifically: More hyperbilirubinemia in the maraviroc arm and also more low-level viremia. Since the population had to have a CD4 > 100 and R5 virus, the bar was not set very high for successful treatment. Could three drugs be required for optimal efficacy no matter what?
- Week 96 data on TDF/FTC + rilpivirine show pretty much what we saw at week 48 (published in this issue of The Lancet): More virologic failure with rilpivirine (especially at high viral loads), but more issues around tolerability/lipids with efavirenz. An open-label study of the two single-pill options using these drugs — Atripla (TDF/FTC/EFV) vs. so-called B-tripla (TDF/FTC/RPV) — is ongoing.
- What happens if you take 311 patients stable on ABC/3TC + a boosted PI and randomize half of them to change the NRTIs to TDF/FTC? Yes, lipids improve and estimated GFR worsens, but there’s also something surprising: fewer virologic failures (3 vs. 11). All low-level virologic rebounds, but still — perhaps the results are telling us something about the relative potency of these two NRTI combinations.
- We’ve known for some time that tenofovir-based treatments reduce bone-mineral density more than comparators, but what are the clinical sequelae? In this large VA-based study, tenofovir exposure was associated with a small but statistically significant risk of osteoporotic fractures (hazard ratio after controlling for everything, 1.12).
- Do boosted PIs increase the risk of chronic kidney disease? Perhaps some of them — notably ATV/r and LPV/r — do, according to these three studies (one, two — abstract says DRV/r, poster didn’t — and three). Is this mediated through their effect on tenofovir levels or some other mechanism?
- Treating acute HIV infection for 48 weeks delayed CD4 decline more than treating for only 12 weeks or not at all. Viral “set point” after stopping treatment was also lower in the 48-week treatment group, with a greater beneficial effect seen if therapy was started closer to seroconversion. Since this was a randomized trial, and since treatment interruption has looked pretty bad in every recent study, are we ready to say that all patients with acute HIV should be treated? I think we’re very close, if not there already.
- Does a regimen’s “CPE” (CNS penetration effectiveness) score improve neuropsychologic function? Not according to this study. Data in this area are so conflicting that one almost needs to be a top performer on neuropsych tests just to keep the studies straight.
- With d4T use being phased out worldwide, the four WHO-recommended initial regimens all consist of tenofovir, 3TC or FTC, and either efavirenz or nevirapine. According to this presentation, TDF/3TC/NVP could be suboptimal, with high rates of virologic failure and emergent drug resistance (especially K65R). Given that this regimen is probably the cheapest and thus the most likely to be implemented widely, confirming its effectiveness is absolutely critical.
A few final words about the conference: The weather was beautiful (one shudders to think what next summer in Washington DC will be like), Rome is extraordinary (so long as you can dodge all the scooters and Fiats zipping around), and I bet all 7,482 participants ate very, very well. Certainly I did.
But did the posters have to be displayed in a converted parking garage?

