An ongoing dialogue on HIV/AIDS, infectious diseases,
June 18th, 2012
ID Learning Unit — Serologic Tests for Syphilis
Diagnosing syphilis is tricky for lots of reasons, including:
- The protean disease manifestations, many of which were best described in older medical literature — and hence not known to people who don’t read words on paper (vs a screen) very often.
- You can’t visualize the bug (Treponema pallidum), unless you happen to have a darkfield microscope nearby — which, if you’re like 99.99% of clinicians, you don’t have at all.
- You can’t culture the bug, unless you use a rabbit — and all I can say about the various techniques of xenodiagnosis is yuck.
- There is a seemingly endless list of available serologic tests, each having a potentially different purpose and all of them carrying an indecipherable acronym.
It’s this last one that is the topic of today’s ID Learning Unit. All you need to know (not everything you could know) about blood tests for syphilis.
There are the screening tests:
- Rapid Plasma Reagin (RPR) — detects antibodies not to T pallidum, but to cardiolipin-cholesterol-lecithin antigens, which are present in active syphilis; which begs the question, what the heck is a “reagin”, and how do you pronounce it? Lots of false positives.
- Venereal Disease Research Laboratory (VDRL) — detects antibodies to the same antigens, just a different technique, and seems to be used much less often; test of choice in the CSF, where it’s believed to be specific (but not sensitive) for neurosyphilis. Of course, how we know that is very mysterious, just like everything about neurosyphilis.
- Treponema pallidum enzyme immunoassay (TP-EIA) — the new kid on the block and, finally, a screening test that actually looks at specific treponemal antigens — hence more specific, fewer false positives. The TP-EIA is replacing the RPR and VDRL as a screening test in many high-volume settings (hospitals, STD clinics, etc); we switched to it at our hospital a few years ago.
Next, there are the confirmatory tests:
- Fluorescent treponemal antibody absorption (FTA-ABS)
- Microhemagglutination test for antibodies to Treponema pallidum (MHA-TP)
- Treponema pallidum particle agglutination assay (TP-PA)
All positive screening tests must be confirmed with one of these confirmatory tests (our state lab does the TP-PA). It’s particularly important for the RPR and the VDRL because, as noted above, these tests measure antibodies to non-specific antigens and have lots of false positives. Hey, weren’t you listening?
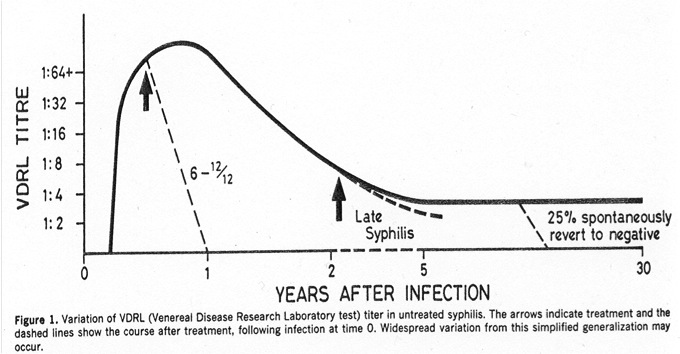 And finally, we bring back a couple of the screening tests to measure disease activity:
And finally, we bring back a couple of the screening tests to measure disease activity:
- RPR (mostly)
- VDRL
Both of these rise shortly after the lesion of primary syphilis (the chancre), then peak during secondary syphilis. Treatment hastens their decline, but some people will spontaneously revert to negative even without treatment (see figure, which is from an ancient but excellent paper, Ann Intern Med 1986;104:368). Which is why the above screening tests (RPR and VDRL) may be unreliable in evaluation of that elderly patient with dementia, and why they don’t really measure disease activity for late manifestations of syphilis. So if you really think it might be neurosyphilis, you should order TP-EIA or one of the confirmatory tests, listed above.
Two small digressions about the RPR and VDRL. First, the units. When positive, these tests are reported in dilutions — e.g., 1:4 is a one-dilution lower (and a two-fold lower) titer than 1:8. Not many of our tests get reported this way (ANA comes to mind as another); in general, it’s only considered a significant change if it’s two dilutions or more — 1:16 goes down to 1:4 after treatment, for example.
Second, they may be falsely negative due to the “prozone” reaction, which does not refer to place to buy auto parts. The prozone reaction occurs when the the antigen and antibody bind together forming a complex, blocking the clumping that labs look for when doing an RPR or a VDRL. If labs are alerted to the possibility of a prozone effect, they can do dilutions to reveal the expected agglutination.
All very simple, right? Hey, it’s topics like this that keep ID doctors in business!
June 17th, 2012
For Inpatients, HIV Medication Errors Common — Then Promptly Corrected
 Several papers have shown that antiretrovirals may be incorrectly prescribed for hospitalized patients with HIV. How do they do at Johns Hopkins — the site of one of the best comprehensive HIV programs in the country (and perennial US News and World Report #1 Hospital in the Universe)?
Several papers have shown that antiretrovirals may be incorrectly prescribed for hospitalized patients with HIV. How do they do at Johns Hopkins — the site of one of the best comprehensive HIV programs in the country (and perennial US News and World Report #1 Hospital in the Universe)?
As described in a new CID paper, investigators reviewed ART medication orders from all hospital admissions among HIV-infected patients in 2009 (702 admissions among 388 patients). In 380 admissions, ART was prescribed on the first day of hospitalization, and in 29% of these, a medication error occurred (145 total). By the second day of hospitalization, however, the error rate had dropped to 7%.
The most common errors were incomplete regimens (for example, prescribing only the PI component and leaving off the NRTIs), but incorrect doses, incorrect dosing frequency, and significant drug-drug interactions were also noted.
Not surprisingly, errors were more common with PI-based components, as these have a wider range of recommended doses, generally require ritonavir, and have more drug-drug interactions. (Plus, let’s face it it’s easier to get right single pill regimens — Atripla one PO QHS — than salvage regimens, pretty much all of which include boosted PIs.) There were significantly more errors on surgical services. The authors attribute the rapid correction of medication errors to their using clinical pharmacy review of medication orders.
These results are certainly consistent with our experience as well: Patients with HIV who require hospitalization often report that on the first day in the hospital, the regimens that they have been taking so meticulously are given incorrectly.
Since hospitalizations among HIV patients are steadily declining, and the vast majority of antiretrovirals are prescribed by a relatively small number of clinicians, we can’t expect hospitalists and medical housestaff to be familiar with these medications. Having them reviewed by a clinical specialist in HIV is critical — a practice we’ve adopted at our hospital as well.
June 15th, 2012
ID Learning Unit — The D Test
I suppose it’s not surprising that we’d follow-up the Etest with the D test, though perhaps if I were being alphabetical, the order would have been reversed.
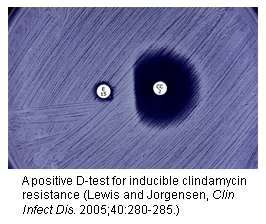 The D test is important, because it screens for a form of clindamycin resistance in MRSA that might otherwise not be detected — the “inducible” kind, which can be associated with treatment failures. About half of MRSA isolates have this form of resistance.
The D test is important, because it screens for a form of clindamycin resistance in MRSA that might otherwise not be detected — the “inducible” kind, which can be associated with treatment failures. About half of MRSA isolates have this form of resistance.
Here’s how it works:
- Take some erythromycin-resistant, clindamycin-“sensitive” MRSA, spread it on a culture plate
- Drop an erythromycin disk on the left side of the plate, and a clindamycin disk on the right
- If there’s a flattening of the zone of inhibition between the two disks, then the test is positive, confirming inducible clindamycin resistance
- Report that bug as clindamycin resistant
First, critical thinkers will wonder why this is important, since we obviously don’t give erythromycin with clindamycin to an actual patient. My big-picture explanation is that it’s a marker for easily inducible resistance even without the erythro being present.
And second, note the critical step of putting the clindamycin disk on the right — otherwise the shape of the zone of inhibition won’t be a “D”, and everyone will be confused because who knows what to call that shape.
(That second part was a joke.)
June 13th, 2012
Questions About HIV Cure, and a Very Funny Quote
 The single case of HIV cure following allogeneic bone marrow transplant is in the news again, this time because of data just presented at “The International Workshop on HIV and Hepatitis Virus Drug Resistance and Curative Strategies” (formerly known as the “HIV Resistance Workshop” — how’s that for rebranding?).
The single case of HIV cure following allogeneic bone marrow transplant is in the news again, this time because of data just presented at “The International Workshop on HIV and Hepatitis Virus Drug Resistance and Curative Strategies” (formerly known as the “HIV Resistance Workshop” — how’s that for rebranding?).
I’m not at the meeting, which is too bad since they often have it in splendid locations.
But from what I gather based on the report, here are the key findings in the study:
- The patient remains off antiretroviral therapy, with a normal CD4 cell count
- He has generously submitted multiple specimens for research analyses at multiple different time points
- Highly sensitive assays of various sorts have been performed at several labs
- HIV RNA has been detected in plasma in 2 (of 4) labs from 3 different time points; levels are lower than those typically seen in virologically suppressed patients on ART
- HIV DNA was detected in a rectal biopsy sample by one lab
- No HIV has been detected in CSF or peripheral blood mononuclear cells
- No replication-competent HIV has been isolated
My gut feeling is that the findings are potentially real, but unlikely to be of much clinical significance if virus can only be detected intermittently by special assays, especially since he’s been off all HIV treatment for more than 5 years.
But they may not be real — we need to remember that a false positive test result is more likely when the test’s sensitivity is cranked up and it’s performed multiple times.
Or as more colorfully put by Doug Richman:
If you do enough cycles of PCR, you can get a signal in water for pink elephants.
And if this interesting presentation tells us anything, it’s that defining success in any study of an HIV cure strategy is going to be very, very difficult.
June 12th, 2012
ID Learning Unit — The Etest
 Every year I attend on the general medical service, so it gives me a chance to work directly with the medical residents — and to brush up on my non-ID-related Internal Medicine.
Every year I attend on the general medical service, so it gives me a chance to work directly with the medical residents — and to brush up on my non-ID-related Internal Medicine.
In exchange for what they teach me, each day on rounds I try to tell them about at least one ID-related thing that they may not know. Since I do this in the spring, they’re awfully sharp. Fortunately, I’ve been doing this ID stuff a long time, so can usually find something.
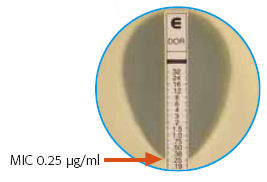
Today’s learning unit was the “Epsilometer Test, or “Etest” — that brilliantly simple way of estimating the MIC of an antibiotic to an organism, by using a drug-impregnated strip that has a gradient of concentration, from high to low. Find where the “elipse” of inhibition crosses the strip, and presto! There’s your MIC.
We take for granted that this method is readily available and, for the most part, clinically valid. It’s worth remembering, however, that when it debuted in the late 1980s/early 1990s, it was considered pretty slick and not entirely trustworthy.
And there’s still some debate about how reliable it is, especially with MRSA.
If you want to learn how to do it, watch this movie.
June 8th, 2012
SPARTAN: Two-Drug, NRTI-Sparing Strategies Continue to Disappoint
 Just published is the cleverly named “SPARTAN” study — spartan because it leaves out both NRTIs and ritonavir — and the results are very interesting.
Just published is the cleverly named “SPARTAN” study — spartan because it leaves out both NRTIs and ritonavir — and the results are very interesting.
Ninety-three treatment-naive HIV-positive study subjects were randomized 2:1 to receive either a two-drug regimen of raltegravir 400 mg BID + atazanavir 300 mg BID, or a standard regimen of TDF/FTC + boosted atazanavir. (The higher ATV dose in the two-drug arm targeted ATV exposures comparable to those achieved by ritonavir-boosting.)
At week 24, virologic suppression rates numerically favored the two-drug regimen (75% vs 63%); as a small pilot study, the trial was not powered for a statistical comparison between the two arms. As has been observed in other studies of integrase inhibitors, the non-integrase group had a slower initial response and appeared to be catching up.
Despite these early favorable results, there were 6 virologic failures in the RAL + ATV group — vs only 1 in the standard arm — and 4 of these failures showed evidence of raltegravir resistance. Notably, all 4 of these patients with resistance had baseline HIV RNA > 100,000, a similar finding to ACTG 5162 (which examined RAL + darunavir/r). Rates of grade 3-4 and grade 4 hyperbilirubinemia were also higher in the ATV + RAL arm. Based on these efficacy and safety results, the sponsor elected to terminate the study.
As I’ve noted before, initial two-drug HIV therapy without NRTIs hasn’t fared well, even when it includes our best drugs. In addition, we still don’t know why these two-drug regimens don’t do as well, especially in patients with high viral loads.
Furthermore, the protective effect that boosted PIs have on the development of NRTI resistance doesn’t apply either to NNRTIs (as shown in ACTG 5142) or to raltegravir (again, ACTG 5162). And though in SPARTAN ritonavir-boosting wasn’t used, it doesn’t appear we can blame PK, as ATV exposures indeed were comparable to those seen with ATV/r dosed at 300/100 mg daily. Do the NRTIs provide some key antiviral component mechanistically? Do we just not have the right two active drugs? Or is there something magic about using 3 rather than 2 drugs, regardless of mechanism of action?
Suffice to say, the results of the fully powered NEAT study — which compares RAL to TDF/FTC, with all study subjects receiving boosted darunavir — will be of great interest, as will a similar study that uses maraviroc instead of raltegravir.
June 6th, 2012
A Fun Internet Poll for ID Nerds
Over on Medscape, one of my ID heroes, John Bartlett, has a new series called, “The Medscape Awards in Infectious Diseases” and it looks like a winner.
Here’s how it works:
The Medscape Awards in Infectious Diseases is a new series that will honor the greatest achievements in the field of infectious diseases during 1980-2012. John G. Bartlett, MD, Professor of Medicine, Johns Hopkins University School of Medicine, identified 8 key categories for these infectious disease awards… Readers will be asked to select the candidate that they believe is most worthy of this title, and then Dr. Bartlett will reveal his personal choice and the reasons for that choice.
OK, I’m game — for two reasons. First, practically anything that has John’s distinctive combination of scholarliness and practicality behind it has got to be good; and second, I am a towering nerd when it comes to Infectious Diseases. I admit it.
So bring on the first category — “Bacterium”.
 The question is, “What do you believe was the most important discovery of a new bacterium during the time period 1980-2012?” Here are the choices, and my thoughts about each one:
The question is, “What do you believe was the most important discovery of a new bacterium during the time period 1980-2012?” Here are the choices, and my thoughts about each one:
- 1980: Borrelia burgdorferi (Lyme disease) — has spawned an entire parallel universe deftly described here.
- 1982: Escherichia coli O157:H7 (hemorrhagic colitis) — posits the eternal question, can a person really learn to prefer a hamburger well-done? Here’s my opinion.
- 1983: Helicobacter pylori (peptic ulcer disease) — one of the true oddities of helicobacter is that ID doctors know nothing about it; it’s like asking an MD about teeth.
- 1986: Chlamydia pneumoniae (atypical pneumonia) — also the cause of coronary artery disease … NOT.
- 1999: Bartonella henselae (cat-scratch disease) — this is my first Ted Nugent citation on this blog.
- 2000: Tropheryma whipplei (Whipple disease) — number of cases most ID doctors personally have diagnosed = zero. Which equals the number of Whipple Procedures they have done, too.
- 2000: Methicillin-resistant Staphylococcus aureus (USA 300 strain) — USA 300 sounds like a motor race, and not surprisingly, it actually is one too.
- 2000: Clostridium difficile NAP-1 strain (C difficile epidemic) — probably the best reason out there to avoid unnecessary antibiotics; I’d be shocked if we’re still using antibiotics as our primary treatment for C diff in 5 years.
- If not listed here, tell us about your choice — I would consider Klebsiella pneumoniae carbapenemase bacteria (KPCs), since they’re a glimpse of a post-antibiotic era, or Streptococcus gallolyticus, because it’s the new name of Strep bovis that I can never remember, or if we’re talking new names in the past 20 years, Stenotrophomonas maltophilia, since it’s such a wonderful mouthful to say.
So my vote?
June 2nd, 2012
Cryptococcal Meningitis Study Stopped — Early HIV Therapy Clearly Harmful
From NIAID, an important clinical trial has been stopped early:
The Phase IV study … was evaluating whether HIV-infected participants hospitalized with cryptococcal meningitis (CM) but not yet taking antiretroviral therapy (ART) would improve their chances of survival if they began ART while receiving CM treatment as inpatients compared with the standard practice of beginning ART as outpatients, approximately five weeks after receiving CM treatment. Two reviews of the COAT trial’s safety and effectiveness data last month by an independent data and safety monitoring board (DSMB) found substantially higher mortality rates among the 87 participants who received early ART compared with the 87 participants who received delayed HIV treatment.
More details on the study design can be found here, and we’ll undoubtedly hear and read additional details on the results in an upcoming meeting and when the paper is published. But since it was stopped after only 174 (out of a planned 500) enrolled, the difference between the two strategies must have been really huge. [See edit below.]
 Until then, we can postulate that ART-induced immune response — IRIS — triggered inflammation, which could have worsened intracranial pressure and other manifestations of cryptococcal disease. Inflammation is just bad news in meningitis. This could explain why crypto and perhaps TB meningitis seem to be the exceptions to the rule that early ART is beneficial with acute OIs — a strategy confirmed now in several clinical trials (ACTG 5164 and these three TB studies: SAPIT, STRIDE, and CAMELIA).
Until then, we can postulate that ART-induced immune response — IRIS — triggered inflammation, which could have worsened intracranial pressure and other manifestations of cryptococcal disease. Inflammation is just bad news in meningitis. This could explain why crypto and perhaps TB meningitis seem to be the exceptions to the rule that early ART is beneficial with acute OIs — a strategy confirmed now in several clinical trials (ACTG 5164 and these three TB studies: SAPIT, STRIDE, and CAMELIA).
Importantly, the study confirms the results of this other cryptococcal meningitis study (which amazingly was led by one of our ID fellows before she started fellowship — how’s that for impressive?), but differs in at least two important ways: First, initial therapy was with the standard-of-care amphotericin B and not fluconazole; and second, the ART was efavirenz- not nevirapine-based. Both of these address at least some of the concerns about the earlier study’s generalizability. A5164 did not find that early ART was harmful in crypto meningitis; however, the number of study subjects with cryptococcosis was relatively small (35 out of 282).
Though the COAT trial was conducted in Uganda and South Africa, the results have immediate applicability to practice world-wide. Early ART in cryptococcal meningitis is clearly harmful, and clinicians should delay starting ART for at least 5-6 weeks in their patients with the disease.
And the study reminds us more broadly that critical studies on the optimal management of HIV-related OIs will necessarily need to come from outside the United States and Western Europe.
[Edit: David Boulware, the PI of the COAT trial, kindly provided a bit more data on the study results. He noted that the “absolute difference in 6-month survival is approx. 15% between the two arms (with ongoing follow up still occurring through October 2012)”, which is perhaps not as big an effect as I had anticipated. However, the message remains the same – early ART after cryptococcal meningitis decreases survival, and should be avoided. He also noted that linkage to outpatient HIV care remains a very important consideration for delayed ART.]
May 30th, 2012
Little Fluffy Baby Chicks Spread Deadly Intestinal Infection
Sorry for the headline, but that was the first thing I thought of when reading this paper just published in the New England Journal of Medicine:
In this report, we describe a prolonged and ongoing multistate outbreak of human salmonella infections primarily affecting young children and linked to contact with live young poultry from a single mail-order hatchery… Because only a portion of salmonella infections are laboratory confirmed, it is likely that thousands of additional unreported infections occurred in association with this outbreak.
To be fair, no one actually died from this outbreak (though 36 patients were hospitalized, most of them 5 years old or younger).
Regardless — it’s hard to imagine baby chicks as vectors for anything, let alone something as unsanitary as salmonella. They’re so cute!
But when you think about it for even a few seconds, why should they be any cleaner than grown-up chickens — which are decidedly not clean.
So the next time you send the kids out to the backyard flock to check for fresh eggs, and they might stop on the way to play with the chicks, pay attention to these wise folks from the CDC, who warn:
Consumers wishing to reduce their risk of illness should practice meticulous hand hygiene and encourage this behavior in children. High-risk groups, including children younger than 5 yearsof age, elderly persons, and immunocompromised persons, should not handle or touch chicks, ducklings, or other live poultry.
So add this to the long (and growing) list of high-risk behaviors.


