An ongoing dialogue on HIV/AIDS, infectious diseases,
August 31st, 2012
“PEARLS” Study a Massive, Impressive Accomplishment
 One of the most frequent criticisms of randomized clinical trials of HIV therapy is that certain patient groups — in particular gay men — are over represented compared to the HIV population as a whole. For example, in the recently published and presented clinical trials of the Quad and dolutegravir, women accounted for < 20% of the study population, despite representing approximately 50% of those living with HIV worldwide.
One of the most frequent criticisms of randomized clinical trials of HIV therapy is that certain patient groups — in particular gay men — are over represented compared to the HIV population as a whole. For example, in the recently published and presented clinical trials of the Quad and dolutegravir, women accounted for < 20% of the study population, despite representing approximately 50% of those living with HIV worldwide.
Now along comes the The Prospective Evaluation of Antiretrovirals in Resource Limited Settings (PEARLS) study, and let no one criticize this trial as not being representative! Conducted in Brazil, Haiti, India, Malawi, Peru, South Africa, Thailand, the United States, and Zimbabwe, the study enrolled 1,571 HIV-1-infected treatment-naive patients, of whom 47% were women. They were randomized to one of three treatments:
- ZDV/3TC + EFV
- TDF/FTC/EFV
- ddI + FTC + ATV
50% were African race, 23% Asian, and 20% Hispanic — remarkably diverse demographics.
A DSMB stopped the study early after observing a greater number of virologic failures in the ATV arm, and a lower than expected number of failures with the other two regimens. Furthermore, while the two EFV arms were comparably effective from a virologic perspective — a finding similar to the prior 934 study, where virologic failures were not significantly different — the safety of TDF/FTC/EFV was significantly better than ZDV/3TC + EFV, particularly in women. Interestingly, women also did better than men on the atazanavir regimen; the reason for this difference is being investigated, and may be related to fact that levels of PIs can be higher in women than men.
The importance of this study is that it greatly strengthens the evidence that TDF/FTC/EFV is truly our gold standard for HIV treatment across a broad range of patients and in multiple settings. It also demonstrates a research collaboration on a massive scale, involving the NIH, several pharmaceutical companies, literally hundreds of investigators at dozens of study sites, and 9 countries!
Additional analyses from this impressive trial are eagerly awaited.
August 28th, 2012
“Quad” Approved by FDA
We now have a third single-pill treatment available for HIV treatment, co-formulated tenofovir/emtricitabine/elvitegravir/cobicistat. From the FDA announcement:
The U.S. Food and Drug Administration today approved Stribild (elvitegravir, cobicistat, emtricitabine, tenofovir disoproxil fumarate), a new once-a-day combination pill to treat HIV-1 infection in adults who have never been treated for HIV infection. Stribild contains two previously approved HIV drugs plus two new drugs, elvitegravir and cobicistat … Together, these drugs provide a complete treatment regimen for HIV infection.
A few quick thoughts about this approval, with the disclosure that I was an investigator on one of the phase III studies that led to its approval:
- The combination — which I’ll continue to call “Quad”, at least for now — is just as active virologically as two preferred initial regimens — TDF/FTC/EFV (the first of the single pill treatments), and TDF/FTC + atazanavir/r (the most commonly used boosted PI regimen).
- The side effect profile was different from TDF/FTC/EFV, with less CNS disturbance and rash, and more nausea; the side effects were quite similar to the boosted ATV/r regimen — except for hyperbilirubinemia from atazanavir, of course — possibly because cobicistat and ritonavir are related.
- The renal issues with cobicistat are potentially tricky, as it causes a 0.1-0.2 mg/dL increase in creatinine that is related to inhibition of tubular secretion of creatinine, and hence does not actually decrease GFR. As a result, the regimen should not be used in patients with impaired renal function, and they were not studied in the Quad trials.
- One potentially helpful number to remember is 0.4, as the small number of patients with tenofovir tubular toxicity in the Quad studies also had an increase in serum creatinine of at least this much — hence distinguishing them from the more benign smaller increase from cobicistat.
- Separate studies of Quad in patients with renal insufficiency and in women are planned (only a small proportion of study patients were women).
The bottom line is that Quad is an effective, very convenient option for initial HIV treatment. I suspect how much traction it gets from providers will depend on their experience in clinical practice related to safety and tolerability, and in the future to pharmacoeconomic factors, as generic antiretroviral strategies will become increasingly available that might challenge use of coformulated regimens.
(How about that name, “Stribild”? Pronounced with a long or short “I”? Wonder what the runner-ups were for that brand name.)
August 25th, 2012
On HCV, These Questions Three
 In the fastest-moving area of ID drug development, answers are eagerly sought to the following questions three:
In the fastest-moving area of ID drug development, answers are eagerly sought to the following questions three:
- What does the bad news on BMS-986094 — formerly INX-189 — mean for other investigational HCV nucleotides? Severe cardiotoxicity, fatal in one case, has ended the drug’s development. Importantly, nothing similar has thus far been observed with the structurally-similar IDX184, but that drug has been placed on “clinical hold” by the FDA. By contrast, the nucleotide GS-7977 is apparently different enough chemically that studies are for now continuing. One take-home message: drug development is risky business.
- When will the alphabet soup of terms used to describe HCV treatment response be abandoned? HCV treatment either works, and you’re cured, or it doesn’t. But because the treatment is so cumbersome and so long, there’s a whole slew of ways to describe how things are going before you get to that point. Let’s see, there’s RVR (rapid virologic response, or no virus detected at 4 weeks); eRVR (extended rapid virologic response, or no virus detected at weeks 4 and 12 — used in this study of daclatasvir); EVR (early virologic response, which really isn’t that early, because it’s week 12, and it can be either pEVR — for “partial” — which means HCV RNA drops by more than 2 log but is still detected, or cEVR — for “complete”, which means it’s undetectable); ETR (end of treatment response, or no virus detectable at end of treatment); and of course SVR (sustained virologic response, now available in many flavors, depending on the week after stopping treatment you want to measure it — 2, 4, 8, 12, 16, 24,etc). For now, with IF/RBV +/- telaprevir/boceprevir and “response-guided” therapy still ruling the day, we’re stuck with this mish-mosh of terms, but I suspect most of this stuff will be irrelevant pretty soon, except for the bottom line — how many are cured? Doesn’t that sound better than “How many are SVR-12’d?”
- So when precisely will these new drugs become available? Seems pretty obvious right now that if you’ve got HCV and can wait for better treatments, you should. Treatment became more effective with telaprevir and boceprevir, but it also got more complicated, toxic, and expensive. Things have to get better, and they will — especially with interferon-free options. Regardless, no one knows exactly when these new drugs will be available for use outside of clinical trials — 2013-2014 a broad estimate — and all kinds of things could hold up their approval (see #1 above). Plus, some patients can’t and shouldn’t wait for better options because they have advanced liver disease. Just this last week, two such individuals came in for evaluation — both with HIV, both with prior treatment failure on IF/RBV, both with Stage 3/4 fibrosis on liver biopsy. Should they wait for daclatasvir, GS-7977, TMC-435, ABT-450/r + ABT-333, etc? Probably not.
August 17th, 2012
Beeper, An Enthusiastic Farewell!
August 17, 2012, is the first day in over 25 years that I left for work without clipping a beeper to my belt.
Yes, our hospital now offers paging through cell phones. Eureka!
As I’ve written before, it was a long time coming. Here are some advantages:
- Spares the embarrassment of wearing a circa-1980s device around non-MD colleagues.
- Portrays a more slimming profile for the fashion-conscious.
- Reduces electromagnetic waves so close to reproductive organs.
- Saves money on AA batteries.
- Diminishes landfill contamination from discarded alkaline cells.
- Decreases the number of items one risks losing, breaking, dropping in the toilet, etc.
- Sheds unfortunate association with drug dealers.
I’m sure there are more, but regardless, I was bound to be an early adopter of a non-beeper life. Yesterday, after passing in the beeper, I almost expected there to be some sort of ritual ceremony, something akin to what they just did in London at the end of the Olympics.
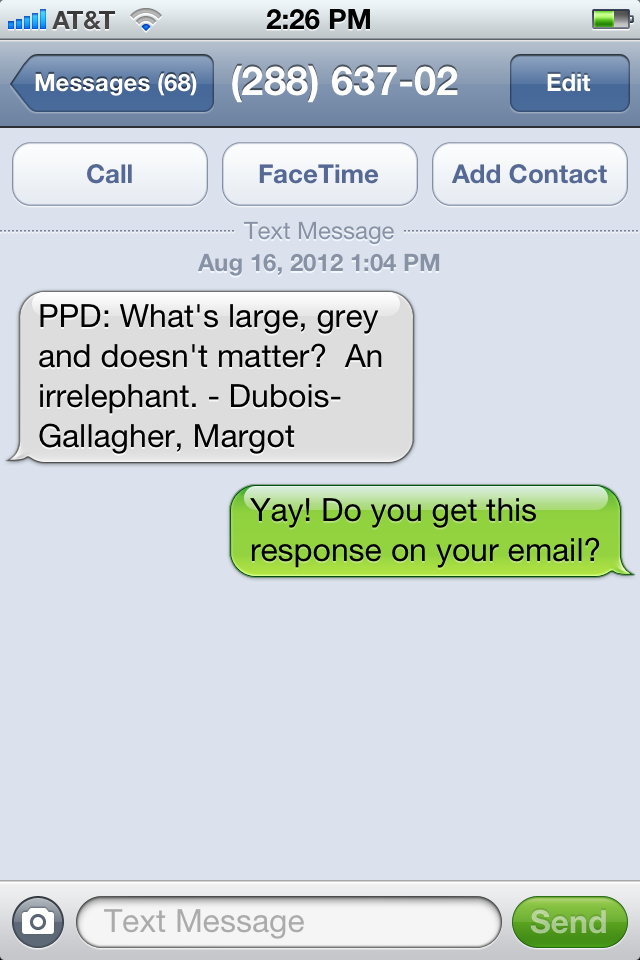
Here then, for posterity’s sake, is the very first page I received on my cell phone, courtesy of our Education Coordinator Margot:
For the record, the answer to my question was yes — she did get my text response in her email. Yet another benefit of chucking the one-way pager.
August 15th, 2012
Brush with Greatness: Atul Gawande
 I was an English major in college, so when my acceptance to medical school (miraculously) arrived, several people gave me books written by doctors about their experience in the medical profession.
I was an English major in college, so when my acceptance to medical school (miraculously) arrived, several people gave me books written by doctors about their experience in the medical profession.
“See,” these gifts implied, “Just because you’re going to medical school doesn’t mean you need to become a science drone. Doctors can write too!”
Sure, doctors can write — but can they write well?
Debatable — confess I’m not a big fan of most books or articles written by doctors. First, the tone in many of them is the very definition of sanctimonious. Second, I often note this strange sameness to the depiction of clinical care. It goes something like this:
- A patient is [choose one or more of the following] doing poorly/unhappy with their care/suffering from a mysterious illness/critically ill/dismissed as a hypochondriac/tired of seeing so many doctors/ready to give up.
- Everyone else in the medical profession is messing up.
- Doctor author comes in and saves the day.
Atul Gawande, a staff writer at the New Yorker, is a wonderful exception to the rule that doctor writers are big show-offs.
He also happens to be a general surgeon at the Brigham, hence the “Brush with Greatness” claim in the title — I get to see him periodically on the inpatient wards.
I’m thinking of him because he wrote a fascinating piece in this week’s New Yorker on how hospitals could learn a thing or two from the Cheesecake Factory, of all places. (Note it’s not an Olive Garden — phew.) The major point is that there’s potentially real value in standardization — better care, lower costs — but that it won’t be easy for individualist doctors and isolated health care systems to adapt.
As usual in one of Atul’s pieces, the tone is just right. It’s been a revelation to read a doctor who writes about clinical medicine in a style that 1) avoids sanctimony and, 2) does not feature a “saved-the-day” story starring MD Superstar.
In fact, when Atul does involve himself in his pieces, it’s usually either as interested observer (such as this wonderful and truly heartbreaking description of modern aging), or to describe his own failings as an MD — trouble with an operation, an erroneous diagnosis, a sense of feeling overwhelmed. These disclosures are inevitably followed by reflections on how he might improve — how very human and appealing.
My favorite part of the current New Yorker piece juxtaposes the precision and lack of waste on the food prep lines of the restaurant with the chaos of a brief hospitalization for an elderly woman after a fall. The woman, who has Alzheimer’s, is the mother of the Regional Manager of the Cheesecake Factory branches here in Boston:
The doctors ordered a series of tests and scans, and kept her overnight. They never figured out what the problem was. Luz [the Regional Manager] understood that sometimes explanations prove elusive. But the clinicians didn’t seem to be following any coordinated plan of action. The emergency doctor told the family one plan, the admitting internist described another, and the consulting specialist a third. Thousands of dollars had been spent on tests, but nobody ever told Luz the results….
Luz’s family seemed to encounter this kind of disorganization, imprecision, and waste wherever his mother went for help. “It is unbelievable to me that they would not manage this better,” Luz said.
If you do any inpatient medicine at all, this is all too recognizable a scenario, and frankly quite painful. And, as usual in Atul’s pieces, a real motivator for us to get better at what we do.
Hey, that would make a good name for a book!
August 13th, 2012
A Poll: Clintons vs Bush
Got this email recently from a former colleague who now does mostly international work:
Hey Paul — nice recap of the IAC conference. But I was wondering if you’d forgotten about someone very important when you wrote, “I can’t think of any major politicians who have done more for HIV than the Clintons.”
Um, how about George W. Bush?
Thanks for considering,
John
It’s a fair point, and I confess I didn’t think of Bush when I wrote that sentence — but should have. The impact of PEPFAR has been absolutely huge (recent summary here), and I’m nearly 100% sure when the history books are written about Bush II’s presidency, this will stand as his greatest accomplishment.
(But then I would think that.)
Meanwhile, the Clinton Foundation and Hillary’s advocacy for HIV patients while Secretary of State are also very impressive.
So what’s your view?
August 9th, 2012
New PrEP “Guidance” Released by CDC
The CDC issued its second “Interim Guidance” on the use of tenofovir/FTC as pre-exposure prophylaxis for prevention of HIV, this time for prevention of HIV in heterosexually active adults. The rationale?
Since January 2011, data from studies of PrEP among heterosexual men and women have become available, and on July 16, 2012, the Food and Drug Administration (FDA) approved a label indication [for TDF/FTC] for reduction of risk for sexual acquisition of HIV infection among adults, including both heterosexuals and MSM. This interim guidance includes consideration of the new information and addresses pregnancy and safety issues for heterosexually active adults at very high risk for sexual HIV acquisition that were not discussed in the previous interim guidance for the use of PrEP in MSM.
I added the emphasis to the above last sentence, as it’s critical that clinicians realize that PrEP with tenofovir/FTC would not be an appropriate preventive intervention for the vast majority of sexually active heterosexual adults in the United States, in whom “community risk” of HIV acquisition is exceedingly low.
That much is pretty clear.
But one area of controversy that has already arisen in clinical practice is whether PrEP is appropriate for serodiscordant couples in whom the infected individual is already on suppressive antiretroviral therapy. It’s an unanswered question — if the risk of transmission is extremely low in these couples but not zero, should PrEP still be considered?
Aside from these small studies (here and here) of its use as part of conception strategy for HIV negative woman who desire pregnancy, we have little if any available data about whether PrEP is worthwhile in this context. The guidance does say, “PrEP use may be one of several options to help protect the HIV-negative partner in discordant couples during attempts to conceive.”
But what about the far more common scenario, outside of conception?
August 8th, 2012
Must-Read Piece: “Imagine a World Without AIDS”
 With all the hoopla at last month’s International AIDS Conference about ending AIDS and curing AIDS and bringing us an AIDS-free generation, there was plenty of ink spilled on the topic.
With all the hoopla at last month’s International AIDS Conference about ending AIDS and curing AIDS and bringing us an AIDS-free generation, there was plenty of ink spilled on the topic.
Ironically, the attention the meeting received was inversely proportional to its scientific content, which was actually fairly light on a content-per-day scale. The meeting probably could have been condensed into 3 days, at least as far as the research findings go.
Regardless, I’d challenge you to find anything better on the topic of how much has changed in HIV care than Danielle Offri’s “Imagine a World Without AIDS,” published the week of the conference in the New York Times.
Dr. Offri did her residency in the early 1990s — I wrote about that grim period here — and worked at Bellevue in NYC, so certainly saw the US epidemic at its absolute worst:
The utter relentlessness of the disease pummeled the doctors-in-training as well. It felt as if we were slogging knee-deep in death, with a horizon that was a monochrome of despair. Witnessing your own generation dying off is not for the faint of heart… If you’d grabbed a random intern toward the end of my residency in 1995, and asked her if she could envision the headline “The Beginning of the End of AIDS” in less than 20 years, she would have simply stared uncomprehendingly at you with bleary eyes.
Bottom line is that if you’re a fan of great writing, do yourself a favor and read the full piece.
July 30th, 2012
2013 CROI Dates and Location: Feb 28 – March 6, Atlanta (Probably)
From the Georgia World Conference Center calendar:
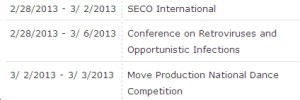 Note that this is not confirmed. But it looks like we’ll be sharing the center with an optometry education company and a dance competition. That should be fun.
Note that this is not confirmed. But it looks like we’ll be sharing the center with an optometry education company and a dance competition. That should be fun.
(Hat tip to an unnamed academic ID/HIV physician for the info, because you won’t find it here — yet.)
[Edit: now confirmed, March 3-7, Atlanta.]