An ongoing dialogue on HIV/AIDS, infectious diseases,
June 11th, 2013
Both Simeprevir and Sofosbuvir Likely Approved by 2014 — Clinical/Ethical/Pharmacoeconomic Dilemmas Loom
 As expected, simeprevir, and now also sofosbuvir, are being given “priority review” by the FDA.
As expected, simeprevir, and now also sofosbuvir, are being given “priority review” by the FDA.
With the 6-month rule under the Prescription Drug User Fee Act — usually just said as “pah-DOOF-ah” — that means there’s a good chance we’ll have both of these anti-HCV drugs some time in late 2013.
Which also means HCV treaters will soon face a major dilemma regarding management of HCV genotype 1.
For those not following this field closely, here’s the deal:
- If approved, both simeprevir and sofosbuvir will be indicated for treatment of HCV genotype 1, but only in combination with pegylated interferon/ribavirin. (Sofosbuvir approval is also expected for genotype 2/3, just with ribavirin.)
- Both will be one pill once daily. Simple!
- Both will cost a lot. Reminder, a course of telaprevir is $50,000, and these two new drugs are better.
- The interim results of the COSMOS study, presented at CROI this year, showed a >90% cure rate in 80 prior null responders (no cirrhosis) who received simeprevir and sofosbuvir together for 12 or 24 weeks, with and without ribavirin. There was no interferon in this study. (The full slide set of the presentation is on NATAP.)
So if these two drugs are both approved as expected, one could easily make the case that the best treatment for HCV genotype 1 — in terms of efficacy, safety, tolerability, pretty much everything except drug-drug interactions and cost — will be the COSMOS regimen of simeprevir and sofosbuvir, with or without ribavirin. And emphatically without interferon.
And that, my dear friends, is off-label use.
So let’s get inside the head of the various players in this potential drama to imagine what they’ll be thinking.
It’s January, 2014, and a patient previously treated with interferon/ribavirin for 48 weeks who relapsed is coming in to review his new treatment options. Here are some potential thought balloons:
Patient: I just want what works best. Sure would be nice to avoid all those nasty interferon side effects I had the last time I was treated.
Provider: I’d like to prescribe simeprevir plus sofosbuvir to avoid all those nasty interferon side effects the last time he was treated. I have some uncertainty about whether to include ribavirin, and whether to treat for 12 or 24 weeks.
Provider’s RN/PA/NP/PharmD who manages all HCV treatment: Sure will be nice to avoid all those nasty interferon side effects that happened the last time he was treated.
Patient’s insurance company: Both simeprevir and sofosbuvir? Two DAAs? You gotta be kidding me — we’re not paying since the regimen is not FDA-approved. Here’s a toll-free number for you to call and argue the case, but the estimated hold time is longer (by a factor of 10) than trying to call an airline to get a flight changed during a blizzard.
Phamaceutical company: We invested a gazillion dollars to bring these drugs to market. Plus, if you consider the net societal savings by curing HCV and avoiding cirrhosis, liver failure, hepatocellular carcinoma, liver transplants, secondary transmission etc, the price is justified.
Activists: The best treatment should be available for all!
Academic MD expert who has been asked to comment, but isn’t actually seeing this patient (or any patient) today because he/she is writing grants and papers, or traveling: The sample size of the COSMOS study is too small to influence clinical practice.
Of course, this will all settle out once there are multiple non-interferon HCV treatment options out there.
I’m an optimist.
June 6th, 2013
ID Learning Unit — Aminoglycosides
 You young whippersnappers out there may not believe it, but we once used aminoglycosides all the time — literally every day on inpatient medical and surgical services, especially in the ICUs.
You young whippersnappers out there may not believe it, but we once used aminoglycosides all the time — literally every day on inpatient medical and surgical services, especially in the ICUs.
They were an inevitable part of “triples” (e.g., amp/gent/clinda), a broad-spectrum combination given to almost every critically ill patient way back when — think right around the time Pac-Man was state-of-the-art and George Bush Senior was President, saying he didn’t like broccoli.
(It’s just an association with the 1980s/early 1990s I’m after. How’d I do?)
Over the years, aminoglycoside prescribing has rapidly and progressively declined — there are other less toxic antibiotics that do the same thing — so these drugs are unfamiliar to many clinicians today.
But they still have their place, so here is brief refresher unit.
Let’s start with a table comparing the four agents in most common use, and finish with some factoids that could be clinically relevant — or at the very least quite helpful when trying to impress your friends. First, the table:
| Drug | Principal Indication | Distinguishing Characteristic Compared with Others |
| Gentamicin | Used in combination with ampicillin or penicillin or vancomycin for treatment of serious infections due to enterococcal (and some streptococcal) infections. | Least expensive. |
| Tobramycin | Used as inhalational therapy in patients with cystic fibrosis, bronchiectasis, and other conditions associated with recurrent bacterial respiratory tract infections. | Most active in vitro versus Pseudomonas aeruginosa. |
| Amikacin | Used as part of empiric broad therapy in patients with known highly resistant GNR infections, or in urinary tract infections with no oral alternatives. | Broadest antibacterial spectrum. |
| Streptomycin | Used as part of second-line combination therapy for tuberculosis and other mycobacterial infections. | Vestibular toxicity a particular problem. |
Now for the factoids:
- These are relatively toxic antibiotics, and patients receiving them should always be monitored closely. Renal, cochlear (hearing), and vestibular (balance) toxicity are the main concerns, and the big problem is that the side effects may be irreversible if the drugs are not stopped promptly.
- The risk factors for these toxicities are exactly what you’d predict. Older age. Impaired renal function. Other nephrotoxic drugs. Critical illness. Higher doses. Higher levels (especially trough concentrations). Longer duration of therapy. Genetic predisposition. You get the idea — can you say “multifactorial”? There, now you can charge for the renal consult you just did on that 87-year-old woman with diabetes and hypertension, taking NSAIDs, who received a course of gentamicin and now has a creatinine increase.
- Monitoring drug levels is essential. The problem is that even when they are “perfect,” end-organ toxicity still occurs. Oh well, it’s at least less likely to happen when toxic drug levels are averted.
- Extended-interval dosing is probably more effective than multiple-day dosing. It might be less toxic, too. (Data are mixed.) The killing of bacteria with aminoglycosides is concentration-dependent, and there’s also a nice post-antibiotic effect even after the drugs are cleared. As a result, we preferentially employ extended-interval dosing when treating serious GNR infections, under the careful guidance of our hospital pharmacy which uses the Hartford nomogram method. Note it’s not appropriate for several patient groups, including those with changing renal function, CF with pseudomonas (see below for clarification), or for endocarditis. [Edit: Extended interval dosing IS used for CF, but the doses are way higher, and the nomogram does not apply.]
- When aminoglycosides are used for endocarditis, use low-dose “synergy” dosing. In enterococcal and certain streptococcal endocarditis cases, you just need low levels of gentamicin — just enough to be detectable on trough measurements. You know, the vermouth in the very dry martini.
- With Staph aureus infections, resist the urge to “just add gent.” Every ID doctor has been there — consulted on a case of Staph aureus bacteremia that won’t go away, despite what should be appropriate therapy. In vitro, Staph aureus is killed faster by combining a beta-lactam with gentamicin than with the beta-lactam alone, so adding gentamicin sounds like a good idea. The problem is that people are vastly more complex than these lab experiments — for example, humans have kidneys and ears — and there has never been a clinical study showing a net clinical benefit from this practice to “just add gent.” With MRSA, we even have the opposite — that adding gentamicin to vancomycin worsens outcomes. So don’t do it. (Possible exception: Staph aureus prosthetic valve endocarditis. Maybe. But call the surgeons anyway.)
- Some gentamicin-resistant enterococci are still sensitive to streptomycin. But faced with using streptomycin for this indication over the past few years, I’ve gone with the ampicillin/ceftriaxone alternative combination instead of amp/streptomycin because: 1) Data are accumulating that this combination actually works; 2) Monitoring streptomycin levels in real-time is all but impossible in most hospitals today.
- Neuromuscular blockade is another complication of this drug class. It’s rare, but it happens — particularly a concern for patients with Parkinson’s or myasthenia gravis, or who received certain paralyzing anesthesia agents. Think of this when, in the ICU, a previously critically ill patient who was paralyzed for intubation is slow to regain muscle strength after receiving an aminoglycoside.
- The only indication for systemic aminoglycoside monotherapy is treatment of urinary tract infections resistant to other antibiotics. This has become a relatively common indication for these drugs, in particular amikacin, due to rising rates of quinolone resistance in GNR. But these are infamously poor drugs for treatment of pneumonia and abscesses, as low pH environments reduce aminoglycoside activity; in addition, single-drug therapy with aminoglycosides in fever and neutropenia was suboptimal. You wouldn’t do these things anyway.
- A trivia question: What rare infections still list aminoglycosides among preferred first-line treatments? Answer: tularemia, brucellosis, plague. Now you’re prepared!
Last, an observation with no apparent explanation: Back when we used quite a bit of gentamicin at our hospital, the doctors all abbreviated it by saying “gent” (one syllable), while most of the nurses said “genta” (two syllables).
Figure that one out.
May 31st, 2013
Fecal Microbiota Transplantation — Try This At Home?
As noted before, the FDA says that an investigational new drug (IND) application is required for therapeutic use of fecal microbiota transplantation.
The practical effect of this decision, at least at our institution, is to stop providing this service — it’s on hold pending those “internal discussions” planned by the FDA on the regulatory issues surrounding the procedure.
Which is why reading this anecdote from Mike Edmond, an ID doctor in Virginia, came as no surprise:
I was scheduled to perform a fecal transplant on a patient this morning, but notified her a few weeks ago that we could not proceed because of the new ruling. She presented to clinic this morning and informed me that she had performed the transplant at home a few days ago. And she was happy to report that she was feeling much better! As it turns out, I have at least 3 more patients in the process of preparing for self-administered fecal transplant at home.
He goes on to say that instructions for doing it home “are readily available on the internet,” which I’m sure is true but am too squeamish right now to check.
(OK, I did it — here is a comprehensive and detailed guide.)
(Fooled you.)
So here’s the irony: FDA’s decision to require an IND will undoubtedly drive quite a bit of FMT into peoples’ homes — where it is completely unregulated. Sigh, unintended consequences are so annoying …
Note that some clinical sites have in fact an IND from the FDA to continue FMT, so it’s worth checking around your local hospitals.
And for those of you who didn’t click the above link, here it is:
May 29th, 2013
The New SARS-Like Coronavirus (MERS-CoV), and What To Do When You Don’t Know Anything About The Latest Outbreak
From one of my close friends — a non-MD — comes this alarming video (sorry, can’t remove the preceding ad).
Concerned? Terrified? I bet your department is buzzing about this.
Um, not quite — especially since, among the 49 cases in the world (apparently there are 5 more than the WHO reported), exactly zero have occurred thus far in the United States. As of May 29, 2013, it hasn’t even cracked the front page of the CDC site.
Is MERS-CoV potentially of great concern? Of course. The WHO response seems right, especially with the parallels to SARS.
But do we garden-variety ID specialists know how serious it will be on a global basis? Of course not. As with the first SARS cases, the first anthrax cases, the first West Nile cases, the first hantavirus cases, even the first AIDS cases — we really don’t have enough points on the graph yet to make any sort of confident predictions.
And from a practical perspective, the clinical unfamiliarity doesn’t help. If someone walked into our emergency room tomorrow with fever, cough, and respiratory symptoms, would we know how to distinguish MERS-CoV from the hundreds (OK, thousands) of other causes of similar illnesses?
Initially, not a chance. The denominator of people with these complaints is just too gargantuan. It will probably take someone with a particularly severe respiratory illness, along with the appropriate exposure (“He just returned from a 10-day business trip to Riyadh”) for an astute clinician to make the connection.
So how should we ID doctors, who are supposed to know everything, respond to these emails in the interim? Stay informed efficiently by consulting these sites:
- ProMed-mail (outbreak updates from your ID colleagues/friends around the world).
- The always-fascinating CDC Outbreaks page, plus it’s coronavirus-specific info.
- Some non-MD news source — I like Google News.
And in response to queries from friends, family, and media, break out those three magic words doctors are often so reluctant to say — “I don’t know” or, if you want company, “We don’t know.”
Because it’s the truth.
(Also posted on WBUR’s CommonHealth site.)
May 21st, 2013
ID Learning Unit — “Isolator” Blood Cultures
Here’s a little secret about those brilliant ID consults we do on patients with mysterious fevers:
Sometimes we don’t know what’s going on either.
I know, I know — shocking.
But now that the secret is out, I can tell you something we do know, and that’s how to recommend lots of tests — the more obscure, the better. Including a particular favorite, isolator blood cultures. If you do inpatient medicine, chances are you’ve had an ID consultant recommend these and may even have ordered them without knowing precisely what they are, or more importantly, or how they differ from the regular blood cultures.
So here’s the story on isolator blood cultures — “isolators” for short — almost in plain English:
- Blood is collected in a sterile fashion.
- Instead of going into regular blood culture bottles, the blood is put into special tubes containing a chemical that lyses (explodes) both red and white blood cells, “releasing” intracellular organisms.
- In the micro lab, the tubes are centrifuged to concentrate any bugs that might be present.
- The sediment is aspirated and placed on appropriate culture media — e.g., fungal media for “fungal isolators” and mycobacterial media for “mycobacterial isolators.”
- Wait. Potentially for a very long time (weeks).
These cultures are also known as “lysis-centrifugation” cultures, which is more descriptive than “isolators” but harder to say.
So when should you order them? There is a literature about the superiority of isolators over standard blood cultures (see video below), but these comparisons are mostly outdated — for example, with advances in blood culture technology (see video below), candida grows just fine in regular blood cultures today.
Furthermore, even though isolators may be superior to standard blood cultures for certain rare infections (e.g., histoplasmosis, bartonella, blastomycosis), in most (all?) of these conditions, use of antigen, serology, or PCR testing has supplanted culture methods entirely.
So that leaves one proven and one possible remaining indication for isolator blood cultures:
- Proven: Diagnosis of disseminated mycobacterial infection (in particular, M. avium complex) in a patient with advanced AIDS or other immunodeficiency. Regular blood cultures are pretty much useless here.
- Possible: Diagnosis of some bizarre, fastidious pathogen (e.g., Malassezia furfur) in a patient with culture-negative endocarditis or a vascular line-related infection.
Which means that most of the time, when your ID consultants recommend isolator blood cultures, it should be for one of these indications.
May 16th, 2013
ID Learning Unit — Antibiotics with Excellent Oral Absorption
 Guaranteed: Every day at a hospital near you, a patient is receiving antibiotic therapy for an infection, and the orders include the following:
Guaranteed: Every day at a hospital near you, a patient is receiving antibiotic therapy for an infection, and the orders include the following:
- A slew of various oral medications, both continued from outpatient care and started anew on admission.
- An intravenous antibiotic.
Even worse, someone may be discharged from the hospital on intravenous antibiotics — risking all the problems of parenteral access (clots, infection, pain, and myriad catheter malfunctions which only happen in the middle of the night/weekends) — when there are multiple oral options that will do the job just as well.
As a result, all ID doctors have encoded deep in their genome a list of antibiotics that can be given orally in place of their IV counterparts, or even more importantly, substituted for something IV on discharge.
Here’s the list, with a miscellaneous comment or two:
- Fluoroquinolones (levofloxacin, ciprofloxacin, moxifloxacin): Yes, we both love them and hate them.
- Trimethoprim-sulfamethoxazole: 1) A double-strength tablet is gigantic, but it also comes in liquid form for the pill-averse; 2) I always ask our ID fellows to tell me precisely (individual drugs and dose) what’s in a double-strength tablet of “Bactrim”, and about half of them know. Do you?
- Metronidazole: 1) I am amazed that after decades of use of this drug, there is still no significant resistance among Bacteroides spp. 2) A ID colleague of mine, who is married to a gastroenterologist, named her bird “Flagyl.” How perfect is that??!!!
- Clindamycin: What a wonderful drug this would be — effective for a whole range of things — if it weren’t for that nasty C diff problem.
- Doxycycline: We’re heading into the “doxycycline deficiency” season here in New England. Start those tick checks!
- Rifampin: You can pretty much guarantee that if a patient needs intravenous rifampin, he/she also needs an ID consult.
- Linezolid: 1) This is the only oxazolidinone antibiotic; 2) I don’t know what “oxazolidinone” means, nor how to pronounce it; 3) I do know that this is by far the most expensive oral antibiotic out there. Wow!
- Fluconazole: We take it for granted today, but the leap from ketoconazole to fluconazole when it first came out was truly gigantic. Sometimes newer really is better.
What’s missing? Most importantly, the entire class of beta-lactam and beta-lactam-like antibiotics — there isn’t a penicillin or cephalosporin that achieves high blood levels with oral administration (amoxicillin and cefadroxil the closest thing), and if there’s an oral carbapenem out there, someone has been hiding it from me.
Finally, as I went through this list on rounds, the residents and medical students were trying to come up with some sort of mnemonic, but were stymied since all the drugs start with consonants. Any suggestions? And did I miss anything?
(Part of a series inspired by attending on the medical service.)
May 12th, 2013
FDA: Fecal Transplants Need Investigational New Drug Application
 Gastroenterologists, ID doctors, C diff-sufferers, and microbiome-obsessed humanoids everywhere were treated to this surprising news recently — a decision by the FDA about fecal microbiota transplantation (FMT). From an email sent by the IDSA:
Gastroenterologists, ID doctors, C diff-sufferers, and microbiome-obsessed humanoids everywhere were treated to this surprising news recently — a decision by the FDA about fecal microbiota transplantation (FMT). From an email sent by the IDSA:
Because fecal microbiota transplantation (FMT) is not approved for any therapeutic purposes, an investigational new drug (IND) application is needed for the use of FMT to treat any disease including C. difficile infection.
More information is on the American Gastroenterological Association site here. This FDA decision was confirmed at a public workshop May 2-3 — “sold out” I’ve been told by one attendee — a meeting which included many interested clinicians and researchers. The FDA plans to hold internal discussions to further refine the regulatory issues surrounding FMT.
I certainly understand the perspective of the FDA, which must consider patient safety as its top priority. But the immediate practical effect of this decision is that getting FMT to the patients who need it — those with recurrent C diff — will now be much more difficult.
And the FDA decision underscores a profound irony in regulatory protocols in our country. A person struggling with C diff can walk into any pharmacy, Whole Foods, Trader Joe’s, GNC, etc, and spend a small fortune on various probiotics, the efficacy of which is marginal at best.
Yet to get FMT, a procedure that has demonstrated quite remarkable efficacy in multiple studies — including this prospective randomized controlled trial — that same patient will need his or her clinician to file an “emergency use” IND application, which is no easy task.
Let’s hope these FDA “internal discussions” proceed expediently. Otherwise, maybe clinicians doing FMT should just start calling it a “supplement.”
May 8th, 2013
HIV Opportunistic Infection Guidelines Updated
Some very hard-working folks at the NIH, CDC, and IDSA have updated the Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents, which are available for review here.
As with the previous versions (the prior iteration is from 2009), the OI Guidelines are comprehensive, exhaustively referenced (184 references for TB alone!), and authoritative. Note that the PDF version weighs in at 416 pages, so I doubt many people will be printing this out and carrying it around in their white coats. Fortunately, for the first time these Guidelines are also available in their entirety on-line in an HTML version, which is undoubtedly how most will access them, and certainly make them easier to update.
After a quick review (no, I have not read all 416 pages quite yet), here are a few of the notable changes, plus a couple of miscellaneous comments:
- There is broadened discussion of when to start HIV therapy in the setting of multiple OIs, in particular tuberculosis. This reflects publication of several pivotal clinical trials — here’s a nice discussion of three of them, to refresh your memory.
- There are updates on treatment of hepatitis B and C; for the latter, strong consideration of deferring therapy is explicitly mentioned for patients with minimal disease given the rapid pace of drug development. This must be the preferred approach for most patients right now, for obvious reasons.
- Diagnosis and management of IRIS — again, in particular for TB — is covered for each OI.
- The structure of the Guidelines is now different, with pathogen-specific tables of recommended prevention and treatment options at the end of each OI section.
- Following ACIPs lead, they endorse the three-vaccine approach to preventing pneumococcal disease — one 13-valent conjugated vaccine and two 23-valent polysaccharide vaccines separated by 5 years; we further discussed it in Journal Watch here.
- Vaccination for HPV is recommended for men and women through age 26. Related: still no formal recommendation to do anal pap smears on either men or women.
- What about the zoster vaccine? Despite this study (presented, not published) showing that vaccination is safe in HIV-infected patients, the Guidelines provide no formal endorsement, only saying it “is contraindicated in persons with CD4 cell counts <200.” (For what it’s worth, I give it to all my patients older than 60 with cd4 > 200.)
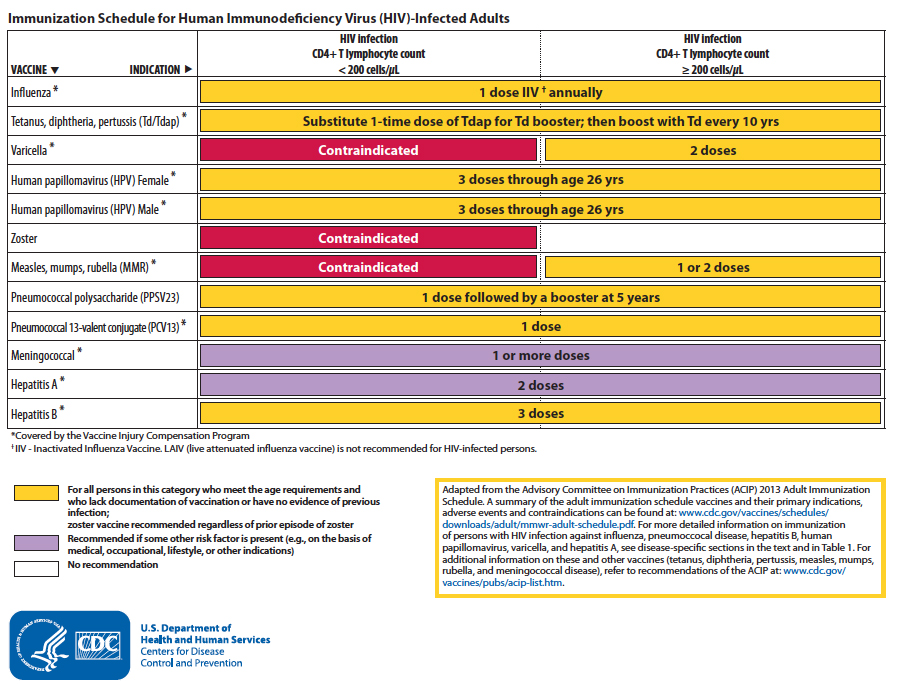
At the end of the document, there are several summary tables as well as this clear and useful figure with the recommended immunizations (click to enlarge):
So I do have a few small quibbles/queries, but they are minor. Namely:
- How did they come up with the order of OIs? It seems to be more or less random. Here’s how it starts — PCP, toxo, cryptosporidia, microsporidia, TB, MAC, bacterial respiratory infections… What the? Undoubtedly there’s a rationale I’m not seeing.
- If you click on the “Endemic Mycoses” link along the left of the HTML version, you get three endemic mycoses (histo, blasto, cocci), plus two that are not generally thought of geographically, cryptococcus and aspergillus.
- Still not much love for beta glucan for PCP diagnosis. With the caveat that we’ve used the test now for years, which makes me far from impartial, it really is quite useful — an HIV+ patient with advanced disease, respiratory symptoms, and a beta glucan > 500 has PCP until proven otherwise.
Note that the panel responsible for these guidelines is inviting comment; just send an email to ContactUs@aidsinfo.nih.gov by May 21, 2013.
I’m sure if we ask nicely, they’ll let us know how they came up with the order for the OIs…
May 2nd, 2013
How to Interpret Medical Breakthroughs in the Mainstream Media
There it is, right in your daily paper, on your tablet or computer screen, or wherever you get your news today — a headline about a great medical breakthrough everyone’s been waiting for:
Scientists on brink of HIV cure
Researchers believe that there will be a breakthrough in finding a cure for HIV “within months”
Yes, I read this exact headline recently. Here’s the full article, published in the English newspaper the Daily Telegraph. It details how some Danish researchers have figured out a way for “the HIV virus to be stripped from human DNA and destroyed permanently by the immune system.”
Furthermore, they are “expecting results that will show that finding a mass-distributable and affordable cure to HIV is possible.”
 By all means, go ahead and read the full piece; you’ve got 20 free reads on the Telegraph website. As a treat, there’s a colorful stock photo too, showing red blood cells floating through some blood vessels, along with a few HIV virions glowing bright green — it’s very Fantastic Voyage-esque, minus Raquel Welch in her scuba gear.
By all means, go ahead and read the full piece; you’ve got 20 free reads on the Telegraph website. As a treat, there’s a colorful stock photo too, showing red blood cells floating through some blood vessels, along with a few HIV virions glowing bright green — it’s very Fantastic Voyage-esque, minus Raquel Welch in her scuba gear.
But return here for a moment, please. I’m going to recommend three simple steps to getting the most from this — and other medical breakthroughs — in the mainstream media.
Step 1: Be a skeptic. As exciting as curing HIV would be, and no matter how much you’d like this to happen, just think for a moment about the plausibility of this story. Are scientists really on the “brink” of curing HIV? If so, why is this only appearing in the U.K. Telegraph? Trust me, this brink-of-cure has not yet appeared in peer-reviewed medical journals or at scientific meetings. And wouldn’t you expect this kind of advance, if real, to show up everywhere in media land? Fire up that Google machine, and see what you can find about it elsewhere — lo, it’s the great following herd, all stampeding after that same U.K. Telegraph story. And importantly, here’s a New York Times piece on the very same general subject — HIV cure — and they don’t even mention these Danish researchers. Sure, the Times misses some stories, but it’s got some pretty impressive Health and Science sections — could they miss this, researchers ON THE BRINK OF CURING HIV, no less? I think not. So perhaps Mr. U.K. Telegraph Science Reporter is exaggerating a bit, for the sake of his story, of course.
Step 2: Don’t be a complete snob — give the story a chance. This is the other side of that same coin. Sure, it’s been a challenge curing HIV, but we’ve got that Berlin patient (now living in Las Vegas, by the way) — he’s cured. And the baby from Mississippi, he/she is cured (sort of). Plus, a whole army of smart HIV researchers actively tackling the problem as we speak. In fact, this very same approach cited by the Danish researchers — stimulating the HIV reservoir with an HDAC inhibitor — is a leading candidate for a viable cure strategy; it’s being looked at by many groups. Hey, why can’t Good Ol’ Ole Søgaard and his team be the first to succeed? The extra funding provided by the Danish government — 12 million Danish kroner! — is further evidence of the soundness of Professor Søgaard’s approach.
Step 3: After all that, land someplace between Steps 1 and 2. Take a deep breath. Read the full piece.
Discard the fluff: Brink of cure; 12 million Danish kroner; expect a cure to be available in months; you can distribute it to millions; it’s affordable, too.
Focus on the facts: Some Danish researchers have some funding to investigate a potentially promising HIV cure strategy; they are testing it in a small number of people; some European scientists may soon be collaborating; we have no actual results yet to report.
After these three steps, all these medical breakthroughs — on HIV, cancer, Alzheimer’s, weight loss, male-pattern baldness, you-name-it — make a lot more sense.
Even if they are less exciting. Enjoy the movie.
[youtube https://www.youtube.com/watch?v=0Sp2bJg_wqI&w=560&h=315]
April 29th, 2013
Data Safety Monitoring Board Closes HIV Vaccine Study — the End of Adenovirus as a Vaccine Vector?
 On Friday, the NIH announced that HVTN 505, a clinical trial of an HIV vaccine using an adenovirus vector, would be stopped based on a finding of futility by an independent DSMB.
On Friday, the NIH announced that HVTN 505, a clinical trial of an HIV vaccine using an adenovirus vector, would be stopped based on a finding of futility by an independent DSMB.
The study had enrolled some 2500 high-risk gay men in the United States. Here are the three key findings leading to this action:
- New infections in placebo arm: 30
- New infections in active vaccine arm: 41
- Among those who got infected, no effect of the vaccine in lowering HIV viral load compared with placebo
While the difference between the two is numerically but not statistically different, it’s clearly in the wrong direction.
Furthermore, it hearkens back to the results of the STEP study — another HIV vaccine trial using an adenovirus vector, Ad5 — that demonstrated an increased susceptibility to HIV infection among participants with pre-study immunity to adenovirus. Though HVTN 505 excluded those who had antibodies to Ad5, the results from HVTN 505 suggest that something else might be going on, something related to Ad5 and protective HIV immunity.
(Confession: I have no idea what that might be.)
As with all studies closed by DSMBs, the detailed data analysis starts in earnest now. And though this outcome is clearly disappointing, it will be fascinating to see what the full results show, and most importantly whether we can learn anything about a way forward in this challenging research area.