An ongoing dialogue on HIV/AIDS, infectious diseases,
October 30th, 2013
HIV Treatment of Serodiscordant Couples: The Home Run, Slam Dunk, and Open Goal in Clinical Research
Just in time for Game 6 of the World Series, my colleague Rochelle Walensky has published a paper in theNew England Journal of Medicine (covered here in NEJM Journal Watch). evaluating the cost-effectiveness of treating HIV-infected individuals in serodiscordant couples.
The results:
In South Africa, early ART was cost-saving over a 5-year period. In both South Africa and India, early ART was projected to be very cost-effective over a lifetime. With individual, public health, and economic benefits, there is a compelling case for early ART for serodiscordant couples in resource-limited settings.
I added the bolding, because in the cautious and scientific style of NEJM, you’re not allowed to do this, but it’s worth emphasizing the main point — everyone with HIV in a serodiscordant couple should be on HIV treatment, regardless of CD4 cell count.
Every so often, a research paper comes up with results that are so staggeringly TRUE that one wonders, what was the controversy? And this is one of them. Take a look at this randomized clinical trial of fecal transplant for relapsing C. diff for a non-HIV example. Or this one on the effect of H. flu immunization in children.
It’s incredibly satisfying, which is why the sports metaphors in the title come to mind.
In fact, when the senior author on the paper, Ken Freedberg, presented these data at the International AIDS Conference in Washington DC in 2012, he was asked by someone in the audience whether he thought the results should “influence policy.”
Like the slugger seeing a pitch right down the middle, the point guard stealing the ball in the backcourt, or the soccer forward beating the goalie who has come out of position, Ken salivated at the chance to answer this question, then, predictably, hit it out of the park. (Or whatever your favorite sport is.)
I don’t remember his exact words, so Carlton Fisk will have to do the talking:
October 25th, 2013
GARDEL Two-Active-Drug Study Not a Game-Changer, but Might Be a Paradigm-Shifter
 Don’t look now, but a two-drug lamivudine (3TC) + LPV/r strategy did just as well as a standard three-drug regimen of two NRTIs + LPV/r. Better, actually, since virologic outcomes were the same and the two-drug regimen had fewer side effects.
Don’t look now, but a two-drug lamivudine (3TC) + LPV/r strategy did just as well as a standard three-drug regimen of two NRTIs + LPV/r. Better, actually, since virologic outcomes were the same and the two-drug regimen had fewer side effects.
Here are the key details about the GARDEL study, presented just this week by Pedro Cahn at the European AIDS Clinical Society meeting, or EACS:
- Study design: Open-label, randomized clinical trial in 426 treatment naive patients.
- Randomization was to 3TC 150 mg BID or 3TC plus an investigator-selected 2nd NRTI; all study participants received LPV/r twice-daily.
- At 48 weeks, 88% of the double-therapy and 84% of the triple-therapy arms had HIV RNA <50 copies/mL
- 1% vs 5% (p = 0.03) in the double- and triple-therapy arms met failure criteria due to no data at week 48 due to stopping treatment or death (mostly stopping treatment; there was only 1 death).
- Rates of virologic failure and resistance were not significantly different between arms.
Ah, but we’re all thinking, what about the high viral load stratum — surely this group would need the extra potency of a three-drug regimen.
But surely we’d be wrong: 87% vs 78% were <50 at week 48, so the difference favoring double-therapy was even greater.
In the “Timing is Everything” category, my recent review of the failed MODERN study of maraviroc + DRV/r included this bit of prescience when discussing why the various two-drug regimens have failed:
What remains unclear is why these two-drug regimens have been so disappointing. Is two drugs not enough? Or maybe just the two drugs tested to date in these clinical studies? Is there something magic about the NRTIs? Or certain NRTIs? (One vote could be for 3TC or FTC, which have been part of every truly great HIV regimen since the late 1990s.)
I added the bolding, because it’s always advisable to highlight when you’re right to help balance out all those times that you’re wrong.
(And believe me, there have been lots of the latter over the years. Just ask my kids. And in HIV treatment, too — remember ddI/d4T/hydroxyurea? What were we thinking?)
So in the HIV world, this study is pretty big news, that much is clear. A two-drug regimen has never done better than a three-drug treatment in a fully powered study.
But will it influence clinical practice? Not right now, I don’t think, which makes this more of a paradigm-shifter than a game-changer. (See, doctors can mobilize business-school cliches too.)
Here are at least four reasons why:
- It’s a three-pill, twice-daily regimen, and commonly used first line regimens are now all easier than that.
- Related, boosted-PI based regimens have lots (and increasing) competition in first-line therapy, especially from integrase-based strategies.
- Clinical practice and treatment guidelines have moved away from lopinavir/r due to study data and clinical experience showing that it has higher rates of adverse effects (GI, lipids) than once-daily atazanavir and darunavir.
- The generalizability of the study results might be limited given that the most common second NRTI chosen by the investigators was zidovudine (54%), followed by tenofovir (37%) and abacavir (9%).
These caveats notwithstanding, the GARDEL study raises several interesting questions:
- Would ATV/r or DRV/r have done as well with just 3TC? Or is LPV/r in this context better? I can’t see why, but of the various two-drug PI plus raltegravir studies, this one with LPV/r seemed to do the best.
- Would once-daily 3TC have done as well? Remember, half-life of 3TC is shorter than FTC.
- What future fixed-dose regimens could we envision, especially as cobicistat-based PI combinations emerge and 3TC is now generic?
- How would a two-drug, 3TC or FTC plus boosted-PI regimen fare as a maintenance strategy? I suspect quite well, though for published data all we have is this small study with ATV/r.
So there you have it, the GARDEL study in all its disruptive innovation glory. You can mail the MBA to my home address.
October 18th, 2013
Back to School: Top Questions from “ID in Primary Care”
 We hold an annual post-graduate course entitled “Infectious Diseases in Primary Care”. In this 2.5 day course, we ID doctors do our best to address the concerns of clinicians on the front lines — those doing primary care.
We hold an annual post-graduate course entitled “Infectious Diseases in Primary Care”. In this 2.5 day course, we ID doctors do our best to address the concerns of clinicians on the front lines — those doing primary care.
And each year, we get some great questions. Like these:
Question: I work at a university health center, and each year we have several hundred visiting students from countries that routinely give BCG immunization. We continue to do PPDs (tuberculin skin tests, TSTs) because they are cheaper, but there are tons of positives. Should we switch to an IGRA (interferon gamma release assay)?
Answer: If ever there were an indication to use IGRAs over TSTs, this is it! Think of the potential money, time, and aggravation you’d ultimately save. Once you switch to IGRAs for those with a BCG history, here’s what you won’t have to do: 1) track down the people who don’t return to have their skin test read; 2) explain to the BCG-immunized with the reactive skin test that we don’t consider BCG in this country when we interpret TSTs (Note: No one who’s received a BCG believes us; they think we’re out of our minds.); 3) get a chest X-ray in those with reactive TSTs from BCG alone; 4) prescribe unnecessary preventive therapy for that same group. Sounds like a good trade off to me.
Question: For skin infections, shouldn’t we double the dose of trimethoprim-sulfamethoxazole [that’s not what he said — he said “Bactrim”] to two double-strength tablets twice daily?
Answer: I’m not sure where this one came from — and it’s a widely held belief — but as far as I know, there are no definitive data supporting a double dose of TMP-SMX for skin infections. Plus, some of the toxicity of TMP-SMX is dose-related, and those “double-strength” tablets are gigantic. Notably, this randomized study used the standard dose (1 DS twice-daily), and this one used variable weight-based dosing. If someone knows why the higher dose is anecdotally so popular, let me know! Which reminds me — what should we call this drug? I generally frown on using trade names unless absolutely necessary, but “Bactrim” (or the less-commonly-used “Septra”) are vastly superior to the mouthful that is the generic name. Some say “cotrimoxazole”, but what is that? Please weigh in by voting in the poll below, and providing your comments. And FYI, I say “trim-sulfa”, but hardly anyone does.
Question: Do you need to check serologies before giving the zoster vaccine if someone says they never had chicken pox as a kid?
Answer: Nope — the good news is that per the zoster vaccine guidelines, we can consider anyone born before 1980 in the United States to be varicella immune. And the last time I checked, everyone older than 60 (for whom the vaccine is indicated) was born before 1980, though you may wish to double-check my math.
Question: Do you recommend repeat testing for C diff after the patient has been treated?
Answer: Certainly not if he/she is improving, as a repeat positive with any of the myriad C diff tests out there is quite common shortly after C diff treatment, and treating improving/resolved C diff with antibiotics is a bad move. So when would you check again? I find repeat C diff testing helpful in someone with a recent episode who’s now having vague symptoms (e.g., not a florid relapse), as a negative result suggests that it’s probably post-infectious GI issues (alteration of normal flora would be the broad generalization of what causes this) rather than a relapse of C diff.
Question: When is next year’s course? I’d love to attend.
Answer: October 1-3, 2014. And we guarantee the New England weather will be perfect and the Red Sox will be in the playoffs.
October 14th, 2013
MODERN Study Stopped: An NRTI-Sparing, Two-Drug Initial Regimen Disappoints Again
 In case you didn’t know, “MODERN” is the clever name for the “Maraviroc Once-daily with Darunavir Enhanced by Ritonavir in a New regimen” trial, which compared TDF/FTC to maraviroc, both with boosted darunavir.
In case you didn’t know, “MODERN” is the clever name for the “Maraviroc Once-daily with Darunavir Enhanced by Ritonavir in a New regimen” trial, which compared TDF/FTC to maraviroc, both with boosted darunavir.
And once again, the NRTI-sparing two-drug regimen comes up short, this time in a fully powered, double-blind noninferiority study.
From a PDF provided by ViiV’s Regional Medical Scientists (the information isn’t on the company’s website yet), here are some pertinent details:
- The study included 791 treatment-naive patients; all had CCR5 virus at baseline.
- At week 48, 72% and 83% of the MVC and TDF/FTC patients had HIV RNA < 50 at week 48 (∆=11%, 95% CI –17.1% to –6.1%; the pre-specified criteria for noninferiority stated that the lower bound of the 95% CI be greater than –10%).
- Thirty-eight patients in the maraviroc (MVC) arm and 13 in the TDF/FTC arm experienced treatment failure.
- No new safety issues were identified in either treatment arm.
- Data are still being analyzed, with further information to come.
As noted before, the bar for successful initial therapy these days is set extremely high, with several recent studies demonstrating 85–90% suppression rates at 48 weeks using regimens with low pill burdens and excellent tolerability. 72% just isn’t going to cut it.
And for the record, here’s a list of NRTI-sparing studies that gave “meh” results at best:
- ACTG 5142 — LPV/r + EFV vs NRTIs + EFV vs NRTIs vs LPV/r.
LPV/r + EFV had high rates of hyperlipidemia; regimen was also cumbersome with lots of GI side effects. - SPARTAN — ATV + RAL vs ATV/r + TDF/FTC.
More treatment failure, more jaundice in the ATV + RAL arm. - PROGRESS — LPV/r + RAL vs. LPV/r + TDF/FTC.
Comparable success rates, but baseline HIV RNA low in the study population; 3 pill, twice-daily regimen. - ACTG 5262 — Single-arm study of DRV/r + RAL.
Unexpectedly high rates of virologic failure (with resistance), especially among those with HIV RNA > 100k at baseline. - A4001078 — ATV/r + MVC vs ATV/r + TDF/FTC
Only 75% virologic suppression rate in ATV/r + MVC arm, with more hyperbilirubinemia than the control group; study not fully powered. - RADAR — DRV/r + RAL vs. DRV/r + TDF/FTC.
63% suppression rate in the RAL arm, vs 84% for TDF/FTC; study not fully powered.
What remains unclear is why these two-drug regimens have been so disappointing. Is two drugs not enough? Or maybe just the two drugs tested to date in these clinical studies? Is there something magic about the NRTIs? Or certain NRTIs? (One vote could be for 3TC or FTC, which have been part of every truly great HIV regimen since the late 1990s.) Do the benefits in bone density for the NRTI-sparing strategies outweigh the lesser virologic activity? (My opinion — no.)
Regardless, HIV clinicians and researchers eagerly await the result of two completed but not yet presented clinical trials — the fully-powered NEAT study comparing RAL to TDF/FTC (both with DRV/r), and the GARDEL study, comparing 3TC to NRTI/3TC (both with LPV/r).
Until then, one should be using NRTI-sparing therapies as initial treatment only in highly select circumstances — and even then, why not include 3TC or FTC?
October 7th, 2013
CD4 Cell Count at Presentation: A Figure with a Depressingly Small Upward Slope
 You know how to make an ID/HIV specialist angry? Frustrated? Sigh loudly?
You know how to make an ID/HIV specialist angry? Frustrated? Sigh loudly?
Tell a clinical anecdote that involves “late” presentation of HIV diagnosis, in particular someone who has been seeking medical care for various ailments for months or even years without getting tested.
You know — it goes something like this:
“He was seen 3 years ago for zoster [or syphilis or pneumonia or thrombocytopenia], and no one sent an HIV test. Now he’s in the hospital with PCP and 10 T-cells.”
It makes smoke come out of our ears because first, it’s so preventable, and second, it doesn’t seem like we’re making any progress.
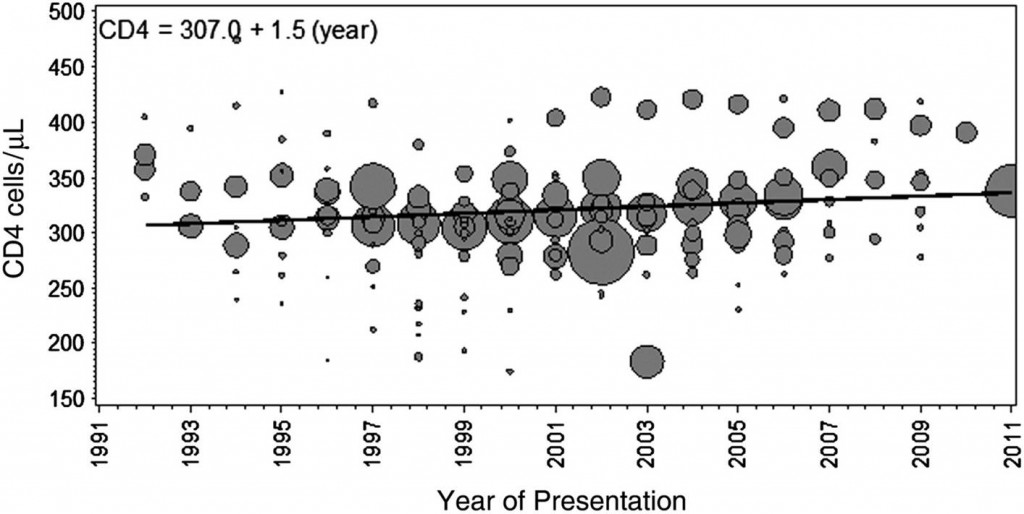
Well, that’s not quite true — we are making some progress, just not very much. In Clinical Infectious Diseases, a new paper summarizes the trend in CD4 cell count at diagnosis for nearly 170,000 patients in studies published from 1992 to 2011. Here’s the punch line:
Mean CD4 cell count at presentation increased minimally by 1.5 cells/μL per year (95% CI, −6.1 to 5.5 cells/μL per year), from 307 cells/μL in 1992 to 336 cells/μL in 2011.
Yes, folks, that’s a 1.5 cell/year increase! Got to show the figure, which no doubt will be much-cited, with considerable hand-wringing:
Note in particular the lack of inflection in the late 1990s — you know, the years when HIV became treatable.
As the authors note, these findings raise significant questions about the “treatment as prevention” strategy, at least if the goal is complete elimination of the HIV epidemic. They also make many of our “when to start” debates completely irrelevant for more than half the newly diagnosed patients — their CD4 is already < 350, often substantially so.
The legal impediments to HIV testing are now pretty much gone, thank goodness. So what is the reason for this continued delay in diagnosis?
September 27th, 2013
Yes! An Economic Justification for ID Specialists
 We’re currently in the middle of fellowship interview season, and I overheard the following conversation between two of my colleagues as they contemplated their upcoming interviewees:
We’re currently in the middle of fellowship interview season, and I overheard the following conversation between two of my colleagues as they contemplated their upcoming interviewees:
ID Doctor #1: He seems like a great candidate — wants to study hospital and community epidemiology of highly drug-resistant bacterial infections, and has already made major contributions to his hospital’s quality assurance program.
ID Doctor #2: Listen to this applicant: She’s been working in [insert resource-limited country here] since junior year in college, even continuing to go there part-time during her residency. She apparently started an HIV screening program in rural healthcare settings that has reduced perinatal HIV infections by 50%!
ID Doctor #1: My next interviewee has his PhD in yeast genetics. I can’t understand his work, but he has three first-author papers in [insert three highly prestigious basic science publications here], and he won the “Top Clinical Resident” at his program last year, proving he’s not just a science drone.
ID Doctor #2: He sounds terrific. But should we ask these young doctors the really tough question about ID fellowship: Will they be willing to work hard in a medical specialty that pays so poorly?
Look, I get it. We just don’t — and can’t — do the obvious things that bring revenues to U.S. medical providers. First, we do essentially no procedures. Second, the very nature of our patient population makes it impossible to churn through high volumes of clinical visits, either in the inpatient or outpatient setting. And third, for those ID doctors who focus on HIV, the demographics of the patients will almost invariably skew toward the indigent.
Now all of this makes us ID doctors, sniff, feel relatively underappreciated, at least from a financial perspective. You should see the red carpet (salaries, office space, advertising, yachts — OK, not yachts) rolled out for our interventional radiologists who treat varicose veins. Meanwhile, we struggle to find funding for the food at our weekly educational conferences.
Is that fair? Of course not.
But what we ID doctors do must have some value, right? Otherwise why does the demand for ID consults seem infinite, increasing all the time? Does our expertise in management of highly complex medical and surgical patients improve patient outcomes and — gasp — even reduce costs?
“Yes!” says this in-press paper in Clinical Infectious Diseases.
The final corrected proof is not yet available, but set aside your “let’s wait for the final published paper” tendencies, and contemplate these incredible findings about those who get ID consults versus those who don’t:
- They had a significantly lower mortality. Is there a more important clinical endpoint? Impossible, kind of like trying to beat 5 Aces in poker.
- They had a significantly lower length of stay in the ICU. We all know that the more days in the ICU, the greater likelihood of badness (a medical condition to avoid) and the higher the cost.
- They had a significantly lower rate of readmissions. Quality assurance gurus love readmissions data.
What’s more, “Patients receiving ID intervention within 2 days of admission had significantly lower 30 day mortality, 30 day readmission, hospital and ICU length of stay, and Medicare charges and payments compared to patients receiving later ID interventions”. I quoted it in its entirety, because how can you improve on that? Better quality and lower costs? Sounds like just what the doctor ordered for our troubled healthcare system.
There’s some potential bias here, since many of the authors are of course ID doctors themselves; plus, we ID docs love Clinical Infectious Diseases; it’s a journal that speaks right to us. But these concerns notwithstanding, I’m hoping this important study will catch the attention of non-ID providers, hospital administrators, and healthcare economists, and that they will subsequently realize that not everything can be measured in RVUs/hour. Maybe they’ll even send some of that varicose vein-procedural revenue our way so we can have sandwiches at our case conference.
Meanwhile, time to continue interviewing these promising young pre-ID doctors for the best specialty in the world.
September 20th, 2013
CROI Abstract Submissions Now Open, and Old CROI Website Still “Lost” in Cyberspace
 HIV researchers can now submit their abstracts to the 2014 Conference on Retroviruses and Opportunistic Infections — or “CROI”.
HIV researchers can now submit their abstracts to the 2014 Conference on Retroviruses and Opportunistic Infections — or “CROI”.
(It rhymes with “soy”, as in “soy sauce”; or, if you prefer, “oy”, as in “oy vey”.)
Further details here. General submission for abstracts closes on October 8.
Meanwhile, people continue to wonder what happened to the now defunct CROI website, retroconference.org (not hyperlinked because there’s nothing there).
It’s not just nostalgia — that site was an invaluable repository of abstracts, posters, and web casts, the go-to place for a staggering amount of pre-publication HIV research. Many have despaired that finding the right reference for their grant and paper now requires all kinds of Google wizardry.
And just check out the abstract links in this Really Rapid Review© — all dead. Blah.
You could try this — but the searches don’t really work, it’s just a tease.
So what’s up? Here’s what it says about past CROI material on on the current site:
We would like to acknowledge that the previous CROI website: www.retroconference.org and the associated materials (abstracts, webcasts, etc), are not available at this time. The CROI Foundation previously partnered with a conference manager that constructed, owned, maintained, and has proprietary rights to the display formats and graphical elements of the website. The former website was taken off line by the former manager. The CROI Foundation fully appreciates how important the content from the prior CROI website is to the colleagues and the community it serves and efforts are underway to recover the content from prior conferences …
So why would someone take this information off-line? Additional details here — some sort of legal fight.
Let’s hope is can be resolved soon, as much of that archived CROI material was very useful.
September 13th, 2013
Clindamycin vs. TMP/SMX for Soft Tissue Infections: A Clinical Trial That Needs Some Marketing
 At ICAAC this week — the ID conference with the most inscrutable acronym out there — Loren Miller from UCLA presented a clinical trial on treatment of skin and soft tissue infections that has widespread clinical applications, yet may receive little if any attention.
At ICAAC this week — the ID conference with the most inscrutable acronym out there — Loren Miller from UCLA presented a clinical trial on treatment of skin and soft tissue infections that has widespread clinical applications, yet may receive little if any attention.
And why is that?
Simply because the drugs (clindamycin and trimethoprim/sulfamethoxazole) have been off-patent for years. Cripes, they’re so cheap that some pharmacies give them away. That’s right — they’re free!
So allow me to market the study a bit.
Here’s the clinical question:
Which is better for uncomplicated skin and soft tissue infections — clindamycin or TMP/SMX?
Clearly the best way to get the answer is to do a randomized, double-blind trial, which is what we have here: Eligible subjects had a skin infection (abscess and/or cellulitis), were not systemically ill, diabetic, or needing hospitalization. If abscesses were present, they were drained.
Participants were then randomized to clindamycin 300 mg three-times daily or TMP/SMX 1 DS tablet twice daily for 10 days, along with matching placebos. (Note: Meets length of therapy rules.)
524 study subjects enrolled at 4 US sites; they had a mean age of 27, with 30% younger than 13. 45% had purulent drainage, and virtually all had I and D as part of their management; the remainder had cellulitis alone. Among those who had cultures, more than half had MRSA; 14% of the Staph aureus isolates had resistance to clindamycin.
14 days after enrollment, 80% of the clinda and 78% of the TMP/SMX group were cured. (About half of the “failures” were really loss to follow-up.) Diarrhea was more common in the clindamycin arm; there were no cases of C diff, and no severe rashes to TMP/SMX.
So to conclude, they’re basically the same.
How to choose? Here are some pros and cons.
- Clindamycin is famously good for beta strep, and active against most (but not all) Staph aureus, including MRSA. But, there’s that diarrhea nastiness, with or without C diff.
- TMP-SMX is active against virtually all Staph aureus, but whether it’s a beta-strep drug depends on whom you ask (many think it isn’t). And of course, it rarely can cause severe rashes and systemic hypersensitivity reactions.
One possible explanation for the near-equal outcomes is that the two treatments failed in different ways — the clindamycin versus resistant staph, and the TMP/SMX for the strep. Since most would get better with no antibiotics anyway (especially those with drained abscesses), these small differences offset each other.
And some might quibble at the dosing of the TMP/SMX — I’ve heard an awful lot of people say with confidence that the right dose for cellulitis is 2 DS tablets twice daily, though far as I know there are no data confirming this (surprisingly) strongly held opinion.
Regardless, kudos to the investigators for getting this study done. It may not yield a glossy advertisement in a medical journal, but undoubtedly will be very useful, cited by numerous primary care, ID, and emergency room providers all over the world.
September 7th, 2013
FAX machines, and the Special Power of the MD Degree
Everyone hates mindless paperwork.
But certain types are particularly annoying, seemingly designed to send you screaming into the night, dragging a broken fax machine behind you as your brain explodes.
Too strong? Take a look at this fax cover sheet I recently received about a patient who had been receiving IV antibiotics at home:
To get at the root of why this particular communication rankles so, let’s do a close reading of the cover sheet — an explication, a detailed description of the prosody and narrative arc, to borrow some words from my English-major days.
Starting at the top, working down:
- It’s from “Health Information Management”: Even though names have changed a lot over the years — hardly anyone was named “Sophia” or “Emma” back when I was a kid — it’s highly unlikely that “Health Information Management” is the name of the person who sent this handwritten note.
- It’s 5 pages. Pages 1 is this cover sheet. Pages 2, 3, and 4 are boilerplate documentation of what has already been done. And page #5 is a task that raises paperwork to a new level of torture — it asks for my signature in 4 ways: 1) Slow signature; 2) Fast signature; 3) Initials; 4) Printed name. That’s a first for me, let’s hope it’s the last.
- It’s “Urgent.” Not just Urgent, but urgent!!! How do we know? Look, the word has squiggly underlining — that means it must be really important. But one might wonder why it’s so important when, as mentioned above, the care has already been given (and, for the record, the patient no longer needs their services, he’s much improved). Could it be that that the definition of “urgent” for this company differs quite substantially from a clinician’s? To a clinician, examples of “urgent” problems include a patient who is short of breath, or bleeding, or having chest pain. For this company, “urgent” means “we want to be paid as soon as possible.”
- They want it “TODAY.” See above, for “urgent”, though now they’ve brought out the all-caps, along with the same squiggly underlining. Clearly, they want me to stop whatever I’m doing and sign the form — 4 times — NOW. One company admitted they stamp all their forms “SECOND REQUEST!!!” — even first requests — as they found this made doctors respond more rapidly to their queries. Sneaky.
- This is all happening by fax. The fax machine is a virtually obsolete form of communication, soon to take its place besides dial phones, signal flags, and smoke signals. But not for home care companies — they love fax machines. They love them so much that the fax number doesn’t just have the squiggly underlining, it also is BOLDED. I can just imagine the office where Health Information Management sits — wall-to-wall fax machines, each carefully monitored, and each shrill fax tone a thrilling announcement that a doctor’s signed (4 times) order is incoming. What joy. (Small aside about fax machines: Over the telephone, “Sax” sounds just like “fax” — which meant back in the heyday of fax machines in the 1990s, I received plenty of correspondence — even faxes — to a “Dr. Fax.”)
- They are unable to accept certain signatures. No stamps. No signatures from NPs or PAs. Nothing from residents. In case it’s not clear, they’ve done something very special with the word “unable” — the triple-whammy of ALL-CAPS, BOLD, and of course our friend the squiggly underline. Must be very important. But think about this for a moment: Can’t residents write orders in the hospital? Prescribe medications to their outpatients? Do various procedures? And PAs and NPs do routine office visits, plus more complex tasks such as covering inpatient medical and surgical services, even performing colonoscopies. So residents, PAs and NPs can do these things — and do them well — but they can’t sign these forms? Maybe the PA and NP curricula don’t include the special class we MDs took on how to sign our name 4 different ways (slow, fast, initials, and print). And that special class isn’t given to just any MD — just those who are board-certified, credentialed, and have completed their post-graduate training. I’d go so far as to say that our ability to sign these forms (4 times) is the true meaning of working to the highest level of our esteemed MD degrees.
OK, I’ll stop now.
August 28th, 2013
Poll: At $14,105/year, Is Dolutegravir Fairly Priced?
 The recently approved once-daily integrase inhibitor dolutegravir is now in pharmacies and, like every new HIV drug, the price — around $14k/year — has generated some controversy.
The recently approved once-daily integrase inhibitor dolutegravir is now in pharmacies and, like every new HIV drug, the price — around $14k/year — has generated some controversy.
For the record, here are the per-year wholesale acquisition costs of the three FDA-approved integrase inhibitors.
- Raltegravir: $12,976
- Elvitegravir: $13,428 (once disentangled from the price of TDF/FTC)
- Dolutegravir: $14,105
If you add the $12 or 15K for the ABC/3TC or TDF/FTC respectively, you get the total cost of initial therapy. So these integrase-based regimens cost more than TDF/FTC/EFV (22.5K) or TDF/FTC/RPV (23.2K), and less than boosted atazanavir- or darunavir-based regimens, which are around 30k.
Now obviously these are all big numbers — HIV treatment is expensive — but the flip side is that it’s so staggeringly effective that it generally meets acceptable criteria for cost-effectiveness given the huge added years of life.
But incremental cost-effectiveness is another matter — meaning, is the additional cost of one drug over another justified, and/or good value?
Here, then, are two opposite perspectives on the dolutegravir pricing:
- The price is fair, according to the U.S.-based Fair Price Coalition. Dolutegravir is an improvement over currently available options, and the slight premium pricing over raltegravir and elvitegravir/cobicistat is justified. Furthermore, the company met with and heeded community advice on price before the release of the drug — a laudable practice.
- The price is unfair, according to the advocacy group HIV i-Base, which is based in London. The makers of dolutegravir went “for gold” in pricing the drug in the United States, and will therefore severely limit the use of dolutegravir in Europe and likely also in resource-limited settings (though prices in these locations are not yet set).
Your thoughts?