An ongoing dialogue on HIV/AIDS, infectious diseases,
June 4th, 2015
A Slightly Less Painful Way to Learn the Three-Letter Abbreviations for HIV Meds
 One of the stupid things about being an HIV/ID specialist is the highly arcane code we use to abbreviate HIV treatments.
One of the stupid things about being an HIV/ID specialist is the highly arcane code we use to abbreviate HIV treatments.
Why was zidovudine originally AZT, and now ZDV?
Why is lamivudine 3TC?
And tenofovir TDF?
Of course there are legitimate biochemical reasons why these are the right abbreviations, but they are lost to most of us who do not have degrees in medicinal chemistry.
Which is why I would like to share that someone I know — a highly brilliant researcher with dozens of papers in high-profile journals, innumerable grants and awards, and many successful mentored investigators — can’t keep these abbreviations straight.
Example: He recently shortened rilpivirine to RLP.
Sorry, “RLP” isn’t going to cut it. It’s imaginative, and original (I’d wager no one has ever used it before), and starts with the right letter, but someone of his stature would certainly be expected to know that it should be RPV.
Of course, he’s not the only one who has trouble with these tricky abbreviations. Medical students and residents despair at learning them, and ID fellows struggle as if they were learning the coagulation cascade. I recently had one smart and experienced PharmD tell me they were “alphabet soup”, and that the abbreviations for HIV meds always threaten to make any lecture on treatment deadly boring.
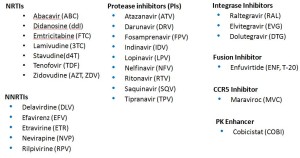
With that in mind, here’s some help. In an effort to help struggling Newbies and Full Professors alike learn these key abbreviations, I’ve pasted below three lists — Advanced, Essential, and Basic HIV Medication Lists.
First, Advanced — the whole kit and caboodle, all 27 medications (click to enlarge):
The secret here is that many of these medications are of historical interest only. You don’t really need to know the lousy old meds that are barely ever used anymore — am thinking of you ddI, d4T, SQV, IDV, DLV…
Sure, many HIV/ID specialists pride themselves on knowing all of them — and even more, that they know the years of FDA approval (of the original drug and coformulations), and the trade names. Most will even know the meds that are no longer available — for example, that ddC was the abbreviation for zalcitibine, a horrible NRTI that had the even more horrible brand name Hivid. Good grief, what were they thinking?
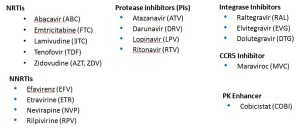
Second, the Essential list — the ones that I’d say are critical to know for most HIV/ID specialists. Note that even though several of these drugs are rarely used any longer as initial therapy (e.g., nevirapine, lopinavir), many patients still are on them and doing great — so you’ll still come across these in practice now and again:
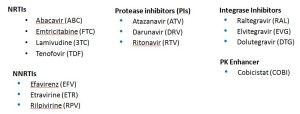
With that second list, we’re down to a mere 18 meds. But it can get even simpler, with the Basic list:
Ah, that’s better — now we’re down to just 14 — the antiretrovirals you’re most likely to encounter actually received by a patient in 2015. One might quibble and say that exclusion of lopinavir and maraviroc was unjust, but hey — we’re talking basic here! Learn these first.
Take it away, Trip.
[youtube http://www.youtube.com/watch?v=uQnDqULwPv4]
May 27th, 2015
START is STOPPED: Study Confirms HIV Treatment Is Beneficial for All, Even Those with High CD4 Cell Counts
The Strategic Timing of AntiRetroviral Treatment (START) study began in 2009, enrolling over 4000 asymptomatic people with HIV and CD4 cell counts > 500, and randomizing them to immediate ART or to wait until the count dropped to 350. Now, from the National Institute of Allergy and Infectious Diseases comes this important announcement:
Though the study was expected to conclude at the end of 2016, an interim review of the study data by an independent data and safety monitoring board (DSMB) recommended that results be released early… Based on data from March 2015, the DSMB found 41 instances of AIDS, serious non-AIDS events or death among those enrolled in the study’s early treatment group compared to 86 events in the deferred treatment group. The DSMB’s interim analysis found risk of developing serious illness or death was reduced by 53 percent among those in the early treatment group, compared to those in the deferred group.
So now we have it — definitive evidence that it’s better to be on HIV treatment than to wait, even for those with normal CD4 cell counts and no symptoms.
It’s worth revisiting, just for history’s sake, why the START study (which opened in 2009) was even done. Remember that once upon a time — OK, a bit more than a decade ago — we tried to wait long as possible before starting someone with HIV who was asymptomatic on antiretroviral therapy. Wait until CD4 = 350? Or even 200? No big deal, provided they had no HIV-related symptoms and were closely monitored.
Seems impossible now — how could we have done such a thing? A bunch of reasons, most (all) of them irrelevant or disproven over time:
- HIV treatment had short and long-term side effects, some of them potentially severe. This was the primary motivation to wait, and actually quite true in the AZT, d4T, ddI, indinavir, etc., era. Of course much less so now. But in the era of lipoatrophy, lactic acidosis, lipoatrophy, high pill burdens, and GI side effects, it made sense to wait if possible.
- We thought there was no downside to waiting, since the immune function (as measured by CD4 cell counts) would return to safe levels after starting ART. Unfortunately this recovery doesn’t always occur, plus we now know there was irreversible loss of immune function based on CD4 nadir.
- Viral replication without loss of CD4 cells was viewed as benign. The potentially deleterious effects of immune activation and inflammation were barely considered, especially before we know the results of the (similarly named) SMART study of intermittent therapy.
- If we treated “early,” then options for therapy would become limited due to resistance — including when patients really need ART (i.e., have low CD4 cell counts). This view was based on the assumption that treatment failure with resistance was inevitable — turns out it’s not. We furthermore didn’t know that the late 2000s would bring a spectacular flurry of drug development for patients with resistant virus. Finally, it ignored the fact that the best way to avoid having a low CD4 was to not let it drop in the first place!
- No clinical trial proved that waiting was harmful for patients with high CD4 cell counts. Over time, there were three randomized studies supporting earlier therapy (one done in Haiti, the clinical outcomes analysis of HPTN 052, and more recently TEMPRANO) — and a fourth if you count the SMART “naive” analysis. However, doubters maintained that most of these data (SMART excluded) were collected in resource-limited settings, and/or used CD4 thresholds that were too low.
- The full benefit of HIV treatment as prevention was not fully appreciated until HPTN 052. Even though indirect data strongly suggested that HIV treatment would reduce viral transmission, it wasn’t until the results of HPTN 052 became available in 2011 that this extraordinary advantage of being on suppressive therapy really hit home, both for providers and patients. This completely changed the dialogue in the clinic — now asymptomatic people with HIV want to be on therapy, for obvious reasons.
The above advances in knowledge have meant that in the United States, HIV treatment guidelines have recommended that all patients with HIV be treated for several years — specifically:
Antiretroviral therapy (ART) is recommended for all HIV-infected individuals to reduce the risk of disease progression … ART also is recommended for HIV-infected individuals for the prevention of transmission of HIV.
It couldn’t be clearer, but to put it in the way familiar to anyone who’s taught from any “HIV 101” slide set, here you go:
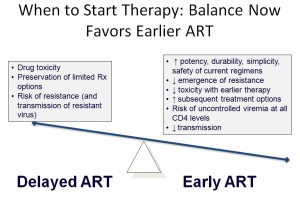
So while the results of the START study are important — and when the full analysis is released, will be fascinating — the study results will not have much of an impact here. Pretty much everyone in care is on therapy already.
The study will, however, have substantial implications globally, where deferring therapy remains a common strategy for patients with high CD4 cell counts.
And score one for Alice Pau and the prescient DHHS Guidelines — they first made this call in 2012. She won’t say, “I told you so,” so I’ll say it for her!
May 21st, 2015
Which Infectious Diseases Do We Fear Too Much? Which Not Enough?
 My friend (and HIV/ID colleague) Mauro Schechter sent me a funny email the other day — from Brazil, where he lives and works:
My friend (and HIV/ID colleague) Mauro Schechter sent me a funny email the other day — from Brazil, where he lives and works:
I just read your post and watched the news clip about Powassan. And you still wonder why we think you Americans are paranoid disease freaks? 65 cases in 12 years in a population of 350 million, and you’re worried??? [The three question marks are his.] Definitely something to get worried about.
Mauro
His last sentence registered 10 out of 10 on the sarcasm meter, and of course Mauro has a point — the risk of getting a severe case of Powassan encephalitis is tiny, even in tick-filled New England. Can’t we Americans find something more appropriate to be afraid of?
On the other side, we’ve had a couple of cases in our hospital, and it really can be quite serious. More importantly, we USAers have a deep-rooted fear of not having enough to fear, and a new tick-related illness fits that void quite nicely — especially on a slow news day.
Which made me wonder — which Infectious Diseases do we fear too much? Which not enough? Here’s a list, compiled with extensive scientific rigor and years (ok, minutes) of painstaking research:
FEAR TOO MUCH:
- Rabies. It’s hard to say that a disease that is nearly 100% fatal and causes thousands of deaths a year can be feared too much, but that’s the bizarre situation with rabies in the United States. The reality is that we have typically 1-2 cases of rabies here each year, and there is no evidence that this is likely to increase anytime soon. Yet think about all those urgent calls, late night trips to the emergency room, and series of rabies vaccine given for possible “occult” exposure to bats. Remember this Canadian study? They estimated that the number of people needed to treat to prevent one case of rabies after bat-in-bedroom-but-no-bat-bite (longest compound phrase I’ve ever written) could be as high as 2.7 million! Not surprisingly, the Canadians no longer recommend rabies vaccine after bats are found in the bedroom. We still do.
- Pharyngitis, possible strep throat in adults. The most feared complication of strep throat is arguably acute rheumatic fever, but: 1) Strep throat is mostly a disease of children and young adolescents, most adults have some viral thing (see below for an important exception); 2) Most acute rheumatic fever occurs in kids as well — even many ID doctors of a certain age (that means older than I) have never seen acute rheumatic fever in an adult; 3) The incidence of acute rheumatic fever has been incredibly low for years in our country, for reasons independent of antibiotic use.
- Conjunctivitis. This ugly, uncomfortable malady makes people really, really scared, and brings out horrible fears of contagion in the school and workplace. You’d think it was a serious, and highly contagious ID emergency. It isn’t.
- Mosquito-related Encephalitis. 2005 was a relatively bad year for Eastern Equine Encephalitis — and there were 21 cases in the whole country. But most years there are only a few, yet this doesn’t stop the near annual news media terror when some mosquitoes test positive. Or a horse dies! Or the mosquito spraying starts! Fear of West Nile Virus means a dead bird can lead to panic, triggering unnecessary calls to the Department of Public Health. Plus there was this bit about canceling high school football. As with rabies, it’s important to acknowledge that these conditions can be incredibly serious, and life threatening — but should they occupy such a big space in our collective fear center?
- Bronchitis. First a little cold, which lingered, but now it’s become bronchitis. Terror, and cue up the Z-Pak!
FEAR TOO LITTLE:
- Influenza. “It’s just the flu”, people say. But people are wrong, pretty much every year. Let’s hope our vaccine gets better — how about one that you only need every five years, not one that needs to be repeated more often than renewing your car’s registration? Progress in the flu vaccine — whenever that happens — will have a transformative effect on community health.
- Clostridium difficile. The emergence of the hypervirulent strain of C. diff should profoundly change the risk vs benefit calculation with any antibiotic prescription. Has it? I know one oral surgeon who will never use clindamycin again, after a “routine” post-operative course caused severe C diff, leading to a colectomy in a previously healthy patient. But how about before the prescription?
- Infectious endocarditis and other bacterial complications of injection drug use. The outbreak of IDU-related HIV in Indiana is appropriately getting plenty of press — HIV is still an incurable disease, much-feared among everyone, including those who inject drugs. But all ID doctors know that the rise in use of heroin has led to a much more pervasive epidemic of endocarditis and other serious invasive bacterial infections. And these are emphatically much harder to treat than HIV, and so much more immediately life threatening. Do people with addiction fear these as well?
- Atypical mycobacteria. There should be a support group for patients with non-tuberculous mycobacterial infections. Pulmonary and non-pulmonary infections from these diverse bugs can be incredibly tricky to diagnose and treat, yet hardly anyone in the non-medical public knows about them. Why is that?
- Fusobacterium necrophorum. The adolescent or young adult with severe exudative pharyngitis, systemic toxicity, and a negative strep test could easily be dismissed as having “only” viral pharyngitis. Yet we now know that a subset will be PCR positive for fusobacterium, the primary cause of septic jugular vein thrombophlebitis (Lemierre’s syndrome), a potentially devastating complication.
- MRSA. New drugs notwithstanding, and even with a decline in incidence (what’s causing that?), MRSA remains the most difficult to treat commonly encountered infection out there. Just ask any ID fellow — what other common infection persists so stubbornly, or recurs so frequently, despite “appropriate” antibiotic treatment?
- Vaccine-preventable diseases of childhood. Self-explanatory.
Would be interested to hear what conditions you think should be on these lists. And as the days grow longer, and we get closer to peak Lyme season, I thought long and hard about where Lyme should go, and concluded it could be on both lists — feared too much by some, too little by others.
See if you can guess why.
May 6th, 2015
An Apology to Subscribers, and Five Random ID/HIV Questions to Ponder
Some of you have been kind enough to enter your email address in the little box on the right side of this page, which gives you a “subscription” to this site. It looks like this:
We thank you for signing up! Delivery is usually prompt and reliable (even during this past winter’s historic snowstorms), and the price can’t be beat (free).
But this week, due to a fascinating technical glitch — text copied from the FDA web site included a prohibited character, a little empty box — no e-mail notification was sent out with the latest post. Our apologies! For the record, the post included various thoughts on the nettlesome problem of fat accumulation syndromes in HIV, and a terrific video from that famous ID specialist John Stewart.
In exchange for this inconvenience, we’re offering a full refund — plus a bonus Five Random ID/HIV Questions to Ponder
- Could this investigational zoster vaccine really be 97% effective? Total game-changer for shingles prevention if it turns out to be this effective and safe.
- Are we going to call the newest azole antifungal “isavuconazole,” or by it’s weird real name, “isavuconazonium?” Strongly hoping the former.
- What percentage of ID doctors know exactly what “MALDI-TOF” stands for? My guess: 54%.
- Has anyone prescribed elvitegravir or cobicistat as individual agents? Probably — it’s a big country. But why did they do this?
- Why did several people in Colorado catch plague from a sick dog? Yikes, a few chewed shoes you expect. But plague?
Speaking of plague …
May 4th, 2015
A Drug for Neck Fat, and Some Thoughts on Fat Accumulation Syndromes in HIV
 It’s not often that a FDA drug approval for cosmetic dermatologists and plastic surgeons will get the attention of HIV/ID specialists, but this past week was an exception. From the FDA report:
It’s not often that a FDA drug approval for cosmetic dermatologists and plastic surgeons will get the attention of HIV/ID specialists, but this past week was an exception. From the FDA report:
The U.S. Food and Drug Administration today approved Kybella (deoxycholic acid), a treatment for adults with moderate-to-severe fat below the chin, known as submental fat … Kybella is a cytolytic drug, which when injected into tissue physically destroys the cell membrane. When properly injected into submental fat, the drug destroys fat cells; however, it can also destroy other types of cells, such as skin cells, if it is inadvertently injected into the skin.
Those of you who don’t practice HIV medicine might not know this, but various fat accumulation syndromes remain a bedeviling problem for our patients. And while I have absolutely zero experience with this new fat-melting injection stuff, I doubt I’m the only HIV specialist who didn’t immediately think of a few patients who could be candidates. Here’s a representative image, courtesy Medscape.
For these patients, the office visit will generally go something like this, almost invariably with someone who has been doing great on treatment for years:
HIV MD: Hi —-, your numbers look terrific — viral load undetectable, CD4 normal, liver and kidney tests fine.
PATIENT: Good to hear! Anything I can do about this weight gain? And this big belly? And look at my neck! I was looking at my drivers license recently, and I look totally different!
HIV MD (Uh-oh, this is a tough one.): Well, there’s diet and exercise.
PATIENT (Does he think I don’t know that? Jeesh.): Yes, of course. What I mean is, are these meds causing me to get fat? If so, should I switch treatment to make it better?
HIV MD (Doing his/her best.): It’s not really the medications themselves, not directly. And switching the meds isn’t going to help. The reason for weight gain on HIV treatment is complicated, and caused by several things … (Various hypotheses outlined, none of them straightforward or easily remediable.)
PATIENT (Oh well. I still think it’s the meds.): OK, thanks.
Now about those “various hypotheses” — below is a short synthesis of what might be going on, by no means meant to be authoritative or comprehensive, but just to get the conversation started:
- Untreated HIV induces a catabolic state. This is particularly the case for patients with advanced disease, where energy expenditure exceeds intake, leading to weight loss. This is the main reason why HIV alone caused wasting, even without a diagnosis of an opportunistic infection. Classic review article here from 23 years ago!
- The main driver of this catabolic state is decreased appetite. In research done in the pre-ART era, a careful analysis of energy intake and expenditure among untreated HIV patients showed that their actual metabolism was often lower than normal — or at most, a little increased — but their food intake was dramatically reduced. The cause of this anorexia is most likely high levels of circulating inflammatory cytokines, such as tumor necrosis factor. This decrease in food intake is often not noted by patients — who may remark, when newly-diagnosed with HIV and very low CD4 cell counts, that they have recently had “successful” weight loss for the first time in their adult lives.
- Effective HIV therapy reverses this catabolic process. Once a person starts on ART, inflammatory cytokines drop, appetite improves, and, as I tell my patients, “the virus is no longer eating any of your food.” The result is not surprisingly weight gain, which is gratifying, even thrilling, especially if there had been serious weight loss.
- The weight gain from effective HIV treatment can be both rapid and too much of a good thing. News flash — there’s an obesity epidemic in most of the world. Not surprisingly, once HIV is treated, our patients become just like the non-HIV population — prone to excessive consumption of processed, packaged, and high caloric junk. In fact, they might be more likely to eat these foods since they are hungry all the time. If they had previously been quite sick from AIDS, then they might also be deconditioned and hence less likely to exercise. And if they’re older, they already have a naturally slower metabolism. Both advanced HIV disease and older age are risk factors for increased weight gain on treatment.
- Rapid and excessive weight gain leads to fat accumulation. In a different form of pathologic “refeeding syndrome” than described in the medical textbooks, the rapid weight gain from HIV treatment can cause fat deposition, most commonly in certain anatomic sites. Probably the best described is excessive abdominal visceral fat, which is associated with increased cardiovascular risk and that big belly the above “patient” described. But abnormal fat accumulation isn’t just limited to abdomen, and every HIV clinician has patients with significant fat deposition in the neck (both anterior and posterior) and upper trunk.
You’ll note that the above list does not cite any specific HIV drugs responsible for this process. That was intentional.
Remember when we used to say that the NRTIs caused lipoatrophy, and the PIs fat accumulation? Turns out we were half right (the first part): the best data we have from randomized clinical trials emphatically does not conclusively implicate one class of drug any more than others. Here’s the most recent of these studies, comparing fat gains with raltegravir, atazanavir, and darunavir-based regimens. I’m sure if you polled a hundred HIV specialists before this study was done, 99 would have bet that raltegravir would be associated with the least fat gain. And 99 would have lost that bet, as all were essentially the same.
So for now, what can we do? A few options:
- Education. We need to do a better job educating our patients about this potential effect of HIV treatment. (Note I don’t say “side effect.”) It’s a return-to-health phenomenon, so the weight gain is a good thing. But a bit of advice about high-quality foods (I’m a big fan of this Michael Pollan book), watching calories, and exercising might do something to prevent excessive weight gain. And we have to be clear it’s not the HIV meds. Switch strategies expressly for this purpose are likely to fail.
- Tesamorelin. The growth hormone releasing hormone analogue with the sonorous name, tesamorelin is FDA-approved for visceral fat accumulation in HIV. On the plus side it clearly does reduce central fat in some patients. On the minus side it’s a
twiceonce-daily injection, it’s expensive, the effects quickly reverse when it’s stopped, and it doesn’t work in all patients. But for a select few, it does the trick. - Make friends with a good plastic surgeon and cosmetic dermatologist. Though several years ago the makers of a facial filler tried to engage HIV specialists in doing these procedures, let’s be frank — it’s best left to the people who do this kind of thing for a living. Some patients will have great results.
- Lobby and advocate. Lots of these treatments aren’t covered by insurance, making them unavailable to those who really need them. That’s a shame, because in severe cases these are highly stigmatizing and dramatically reduce quality of life — they should be covered!
- Research. If there’s a specific cause to this weight gain and fat accumulation problem, let’s see if we can figure it out. Fortunately, there are lots of smart people who continue to study the mechanisms of this process.
Meanwhile, if anyone has experience with this new drug for neck fat, let me know!
And enjoy this video, just because we’ll miss him…
April 22nd, 2015
Seriously — How Much Would You Pay for a Curbside Consult?
Let me start with an email exchange I had with a PCP recently:
Hi, Paul, quick question 😉 This lady, 49 YO woman from Haiti, asymptomatic, totally healthy. Got TSpot done for immigration purposes, it’s positive with negative chest Xray. Treated with INH 6 months in 2001. She travels to Haiti annually so could had been reexposed, though doesn’t report being with anyone with TB. Do I need to treat her again? THANKS!
Carla
To which I responded:
Hi Carla, unless there’s been a significant re-exposure and immunosuppression, no need to re-treat.
Paul
p.s. You know we could start getting paid for these “eConsults.” How much do you think they are worth?
So it’s now Carla’s turn to answer my question:
Priceless!!!! Seriously, not sure, since some questions are more complicated than others. Sometimes it’s straightforward like this, and sometimes you have to review more data so that should be compensated more no?
Carla helped me out here, especially with her first response — priceless indeed. But the second part — where she suggested that this “straightforward” question is worth less a more complex one — hints at why estimating the value of these consults is so difficult.
And it’s important that we figure it out, and soon. With the inevitable move to “value-based” insurance contracts, many ID doctors are getting the push to formalize these clinical relationships. That’s a good thing, as clearly getting advice from a colleague can be a more efficient way to provide quality care than referring everyone for a formal consultation, and it puts a value on what previously was almost always done gratis.
Back to the question in the email — is it so straightforward? Clearly not to Carla, who as a generalist has to manage all medical problems not just ID ones, but to us brilliant ID doctors, sure. An IGRA (the T-Spot) and tuberculin skin test are basically two different ways of looking at the same thing (evidence of immune response to TB), and I’ve already been asked this question (or one like it) several times. Plus, I can touch-type (as I’ve bragged elsewhere, I could crank out over 70 words/minute in my 8th grade typing class), and happened to be sitting at my desk daydreaming about puppies playing baseball when the email popped into my in box.
Even if you account for the distraction factor — it was hard to get that puppy image back — it still took only a few minutes.
And as I’ve calculated before, if paid on an hourly basis, this comes out at a whopping $6/consult, or almost enough for a couple of fancy coffees at Starbucks. If we believe the good news from this latest Medscape salary survey that ID doctors are no longer in the basement when it comes to annual salaries — sorry, pediatricians — we’re only up to $8/consult (add a bagel to your coffee order).
So when it comes to curbsides, if you pay by time/consult, we ID doctors could be in deep trouble — the faster we do it, the less time-value it has, and the less we make. It’s quite different with surgeons — they get paid more with greater experience and efficiency.
Furthermore, though the fee-for-service model is horribly broken for ID as a specialty — no way to win on either volume or procedures — our clinical productivity is still mostly measured by how many patients we actually see. Every curbside we do is potentially a patient visit that didn’t happen. Note again how we differ from surgeons and other procedural specialists — from a revenue perspective, they have an incentive to keep simple non-operative cases out of their clinics.
Finally, the consultant is taking on some non-zero medicolegal risk. Should that be somehow factored into the compensation? In this excellent review — which is very pro-curbside — the author states that courts have consistently concluded that there is no actual medicolegal risk to the person being curbsided provided there is no relationship between him/her and the patient. However, he also accurately states: “Of course, even in the absence of actual liability, there is always a possibility that the consulted physician will be sued for medical malpractice. Although such a physician should ultimately prevail as a matter of law, the entire process is best avoided [emphasis mine].” Hard to disagree!
Other payment models are out there for informal consults, such as getting a flat rate per consult regardless of complexity (how much?), or getting a percentage of your salary covered for being “on call” for this service (what percentage?). And of course many will still have to live with the status quo of getting nothing — gratitude counts for something.
Provided, of course, it doesn’t end with (pet-peeve alert) “Thanks in advance!” A simple “Thanks” is perfectly fine.
Now, how much would you pay?
April 15th, 2015
Does Scientific Language Come Across as Wishy-Washy?
 I had the opportunity to interview author Seth Mnookin recently for a podcast on Open Forum Infectious Diseases, and it was a real treat. He’s Associate Director of the graduate program in Scientific Writing at MIT, and the author of the The Panic Virus: The True Story Behind the Vaccine-Autism Controversy.
I had the opportunity to interview author Seth Mnookin recently for a podcast on Open Forum Infectious Diseases, and it was a real treat. He’s Associate Director of the graduate program in Scientific Writing at MIT, and the author of the The Panic Virus: The True Story Behind the Vaccine-Autism Controversy.
Not surprisingly given his title, Seth thinks a lot about how language conveys information about health and science. While researching the topic of vaccine safety for his book, he discovered the striking difference in how the various sides communicated their message to the public:
When you had an NIH official or a CDC official making a statement and then you had a parent, it almost seemed to me like they were talking in two different languages … So when a doctor or Harvey Fineberg would be on TV and say, “Well based on all of the evidence that we have, we’re reasonably confident that there’s no concern that vaccines cause autism that we know of at this point, etc.” In science that means, “Yeah, we’re really sure about this.” But in English that means, “Yeah we have no idea what we’re talking about and we’re about to find out that actually we’ve been doing something horribly wrong…”
And the parents challenging vaccine safety? They couldn’t be more sure of themselves:
A great example of this is when Jenny McCarthy was on Larry King’s show and she was talking about her child, and she said, “The story that I have about my child is science.” [She also said something almost exactly like this on Oprah.] A point that I try to stress is that actually the plural of anecdote is not data, and that the fact that there is a story to be told about something does not make it true.
In research, we’re taught to be curious, questioning, and skeptical — but this doesn’t play well on the big stage. If the message is as clear as the overwhelming case for vaccine safety and efficacy, we have to just say it.
There’s more interesting stuff in the full interview, including how he chose to tackle this tricky subject, how big of a problem the hard-core anti-vaccine activists actually are right now, and when the police start to take seriously various threats on your life (not kidding about that, unfortunately).
April 8th, 2015
New HIV Treatment Guidelines, and the End of an Era
 The new Department of Health and Human Services (DHHS) HIV treatment guidelines are out, and thanks to skillful direction by Alice Pau, it’s as usual a must-read document — all 288 pages, of course!
The new Department of Health and Human Services (DHHS) HIV treatment guidelines are out, and thanks to skillful direction by Alice Pau, it’s as usual a must-read document — all 288 pages, of course!
There are several major changes, so a good place to start is the all-important “What’s New in the Guidelines” summary page. Some of the biggest modifications come in the “What to Start” section:
- There’s now a more focused list of “Recommended regimens” — it’s down to just 5. Specifically, TDF/FTC plus DTG or EVG/c or RAL (that’s 3), ABC/3TC/DTG (4), and TDF/FTC plus DRV/r (5).
- The regimens that are limited to patients with low HIV RNA are now classified either as “Alternative” — TDF/FTC/RPV — or “Other” (ABC/3TC plus EFV, ABC/3TC plus ATV/r).
- TDF/FTC plus ATV/r is now an “Alternative” regimen, largely due to the results of ACTG 5257.
- TDF/FTC/EFV is now an “Alternative” regimen, largely due to issues of tolerability.
With the caveat that as a member of the Guidelines panel, I can only give you my personal perspective (not that of the committee), here are a few comments on this last one — the demotion of efavirenz from “Recommended” to “Alternative” — which seems to me a pretty big deal.
First the good stuff about EFV, which was approved by the FDA way back in 1998:
- In clinical trials, efavirenz has been better or as good virologically than all its comparators for years and years. I still remember the shock when we learned that EFV creamed indinavir — a potent protease inhibitor, who would have predicted that? — and subsequently it won or tied in numerous head-to-head studies. That success continued until the drug was compared to integrase inhibitors (in particular dolutegravir), but note that rates of virologic failure were still just as low with EFV even in this comparison. And is there any agent that so consistently does well in patients with high baseline HIV RNA and/or low CD4?
- Efavirenz has such a long half life that regimens with the drug are remarkably forgiving, even if people forget to take it every day. It’s so forgiving, in fact, that studies suggest you can do fine taking it only 5 days a week, or at a reduced daily dose. Not that we recommend these strategies, but still — we all have patients on EFV-based regimens who admit that they skip it periodically (usually because of side effects, but that’s a different story), yet they maintain virologic control.
- Although no HIV treatment is cheap, TDF/FTC/EFV is less expensive than most of the other initial regimens we use today.
- Efavirenz (with TDF/FTC or TDF/3TC) is the default initial treatment globally, where it is widely available as a single pill taken once a day. That counts for a lot — obviously the vast majority of people with HIV in the world don’t live here.
So what’s the issue? Why then is it now an “Alternative” rather than a “Recommended” option? In my opinion, it comes down to progress we’ve made in improving side effects. Many choices are available now that are simply easier for patients — and clinicians, who can skip the time on pre-treatment education and management of tricky side effects. Specifically:
- All the clinical trials comparing EFV with integrase-based options demonstrate significantly lower rates of central nervous system (CNS) side effects with the latter. As already noted, in the head-to-head study against dolutegravir, drug discontinuations due to adverse events led to a superior result for DTG. The same thing happened when EFV was compared to RPV — in the low viral load stratum, RPV was superior because it was better tolerated.
- Virtually everyone who starts EFV gets some sort of CNS side effect of varying severity in the first week or two. Not a good idea to start the day (or even a week) before a big presentation, or travel, or some other major life event. In most patients, these CNS side effects diminish rapidly over the first few weeks of therapy. However, a minority still have some residual weirdness going on long term — dizziness, abnormal dreams, morning grogginess. Some learn to live with it and are fine, but others don’t realize how off they’ve been feeling until they stop the drug. (Brief aside — what’s up with the small fraction of patients who choose to take EFV during the day? That always perplexed me.)
- More serious CNS side effects can rarely occur, in particular depression. In this retrospective analysis of four randomized clinical trials, patients randomized to EFV-based regimens had a more than two-fold increased risk of suicide or suicidal ideation compared with those not receiving EFV. And while the absolute risk was overall low, this is a severe enough adverse effect that one should be very cautious about using the drug in anyone with a history of depression. Although observational cohort and claims data have not shown this association, remember that this is a tricky thing to find in such data, and that in clinical practice we avoid prescribing EFV to patients with psychiatric disease.
- Every ID/HIV doctor has had patients who just can’t take this drug, and it’s not from depression. OK, anecdote time — here are a few of mine: The guy who drives for a living who knew immediately he wasn’t as alert on the road taking EFV. The person whose dreams were so vivid that they were essentially indistinguishable from hallucinations (and not pleasant ones). The high-functioning scientist who simply couldn’t concentrate at work. The person (actually a few) with severe rash and fevers. Of course some of the vivid dream stories were pretty funny — my favorite was someone who dreamt that her kitchen had been extensively renovated, including specific selections of cabinets and appliances. Imagine her disappointment when she came downstairs to find the scruffy old kitchen unchanged!
Yes, I still have patients on EFV-based treatment who are doing great, and they don’t want to switch — that’s fine, no reason to do so. But the bottom line is that I haven’t prescribed TDF/FTC/EFV to a patient starting HIV therapy in nearly three years. Too many other good options out there now.
Hey, progress is a good thing!
I did this poll before — now let’s try it again, a year and a half later:
April 3rd, 2015
Melting Snow ID Link-o-Rama
 A few ID/HIV tidbits to contemplate as we go from slipping on ice and snow to dodging the mud:
A few ID/HIV tidbits to contemplate as we go from slipping on ice and snow to dodging the mud:
- Beta-lactam therapy alone is non-inferior to regimens that also cover “atypicals” for hospitalized patients with pneumonia. These results challenge a dogma that has been present for a couple of decades — namely, that all patients admitted with community-acquired pneumonia should get either a quinolone or a beta-lactam plus a macrolide. But is one study done in the Netherlands enough to change clinical practice?
- Flurry of recent papers on the cost effectiveness of HCV therapy: One, two, three, and four, to be exact. Bottom line? A huge oversimplification goes like this — from a societal perspective, the new treatments are for the most part cost-effective and expensive. And, of course, from an individual perspective — meaning you are the individual being treated, or the prescriber — they are a no-brainer.
- Related: Entertaining and informative discussion of HCV pricing, and what’s going on behind the scenes from longtime ID/HIV clinician, researcher, and educator Mike Saag. When it comes to this market, what’s the opposite of transparent? Highly recommended, especially for fans of Get Smart. And if you want some additional info on the world of “Pharmacy Benefit Managers”, read this. (Thanks to Mike for the link)
- “Fourth Generation” HIV screening tests that detect both antigen and antibody can still miss acute HIV. Remember, the duration of p24 antigen detectability during acute HIV is relatively short. Important to think about this limitation, especially when evaluating patients for PrEP — we should have a low threshold to order HIV RNA.
- The outbreak of injection drug use (IDU)-related HIV in Indiana is a stark reminder of how quickly HIV can spread with the right mix of bad ingredients — rising rates of opiate addiction, poverty, and lack of access to clean needles. Notably, in the rest of the US this mode of HIV transmission has become quite rare — here in Boston, I haven’t seen a newly acquired case of HIV from IDU in years. Bad time for complacency on this issue.
- Watch out for ciprofloxacin-resistant shigella in returning travelers. As noted in the report, “shigella is transmitted easily from person-to-person”, which is something of an understatement since some people can get sick after ingesting fewer than 100 bacteria.
- The air near beef cattle feed yards carries antibiotics, antibiotic resistance genes, and resistant bacteria. Yuck, good idea to stay up-wind. Remember, 80% of our national use of antibiotics goes to livestock. Will the programs announced this week by Obama actually reduce this use?
- I recently learned that two of the HACEK organisms have new names: Haemophilus aphrophilus is now Aggregatibacter aphrophilus, and Actinobacillus actinomycetemcomitans is now Aggregatibacter actinomycetemcomitans. Wow. The mnemonic “HACEK” still works, however, standing for Haemophilus species, Aggregatibacter species, Cardiobacterium hominis, Eikenella corrodens, and Kingella species. I’m convinced some of us chose ID as a specialty just so we could know information like this.
Hey, it’s Holy Week. Colored (sometimes green) eggs and ham! Peeps! Matzo balls and brisket! Jelly fruit slices! For those of a less traditional spiritual bent, enjoy this:
H/T to Joel Gallant for the vid.