An ongoing dialogue on HIV/AIDS, infectious diseases,
October 23rd, 2025
What a Difference a Year Makes — with Bonus Halloween Video
At last year’s IDWeek — the annual meeting of the Infectious Diseases Society of America — a group of us veterans in the HIV/ID world wrapped up a busy day of symposia, abstracts, and posters with a lively dinner in Los Angeles.
What do I mean by veterans? If you started your ID training before effective HIV therapy, you qualify: Joe, Trip, Judy, Joel, Jeanne (!!!), me — well, here’s the email I sent after the dinner:
Hi All,
What a fun night! Despite the adventure of getting there in LA crazy traffic (thank you Judy for the lift and your bravery driving in such madness), and the deafening restaurant noise which made us all need to shout, it was a great meal. I awoke today feeling very grateful for having such smart, interesting, and funny long-term colleagues and friends, and optimistic about what’s to come with Jeanne’s exciting new position.
Thanks also to Joel for galvanizing this gathering of us ID docs who did our fellowships in the late 1980s/early 1990s, which was more than 3 decades ago, but who’s counting.
Paul
So what’s the ‘!!!’ doing after Jeanne’s name up there in the second paragraph? That, of course, referred to Dr. Jeanne Marrazzo, who had recently been named Director of the National Institute of Allergy and Infectious Diseases (NIAID), a position she took over from long-time head Tony Fauci. Even though I’ve known Jeanne for years, last year I was a bit star-struck and flattered that she chose to dine with us.
(Maybe she chose us because we all share her love for dogs. Yep, it was that!)
Jeanne spoke about NIAID, the directions she wanted to take it, the challenges our country faced, and just radiated the kind of positive energy that we’d want in this important position. Going from Chief of ID at the University of Alabama to this NIAID head position seemed the right next career step for such a natural born leader. Everything she said exuded optimism, and promise.
Fast forward a year — IDWeek again, this time in Atlanta. You could not imagine a more dispiriting turn of affairs. Right there, in the home city of the CDC, we heard about their laid-off staff and furloughed workers. Barely any CDC officers attended an ID meeting in their hometown — how’s that for irony? Those that did — to showcase their research or to teach us — did so on their own dime, as they’re certainly not receiving a salary or stipend from the organization that they supported for years, sometimes decades.
And what about Jeanne Marrazzo? What’s happened to her?
As has been widely reported, in the year since our dinner she went from celebrated leader to embattled critic. After raising alarms about what she viewed as the politicization of NIAID’s grant process and a growing hostility toward vaccine research, she was placed on administrative leave earlier this year. In September, she and vaccine researcher Dr. Kathleen Neuzil filed a whistleblower complaint alleging retaliation for those concerns. A few weeks later, she was fired — no reason given — but how can we see it as anything other than the silencing of a scientist who spoke up?

(Shared with permission.)
So what a year. From a gleeful dinner in Los Angeles to the sober conference in Atlanta. The same faces, the same field, but the atmosphere flipped upside down by the results of the November election. Last year the energy was forward-looking with a newly minted NIAID director, confident colleagues, and the sense that we were part of something steadily advancing. This year: layoffs, sidelined personnel, “RIFs” — everyone had a story about a grant cancelled, a program blitzed, an international project put on indefinite hold.
And yet — I saw Jeanne at the meeting, and am delighted to say that she’s still bursting with energy and a desire to do something good in the world of Infectious Diseases. Likening herself to a border collie now confined to the house — restless, needing to get out and accomplish some tasks! — she’s looking at numerous options for deploying her various talents. And what a quintessentially on-brand metaphor for herself.
Wherever she ends up will be the better for it, I’m sure of that.
For the rest of us, the times certainly have changed since last October, but the community still felt strong. No, IDWeek 2025 didn’t have the optimistic buzz of last year’s meeting, but nearly everyone I met this year carried the same conviction — that good clinical care, good research, good teaching, and above all, good people, still matter.
Matter a lot.
Now, for that bonus video I promised …
October 16th, 2025
Another Bad Week for the CDC, and a Personal Note
 On Friday night, news broke that more than a thousand CDC staff received layoff notices — including people who track measles outbreaks, analyze data to craft policy, monitor employee safety, and, remarkably, recent Epidemic Intelligence Service (EIS) fellows (the early-career epidemiologists who often show up first when something alarming appears).
On Friday night, news broke that more than a thousand CDC staff received layoff notices — including people who track measles outbreaks, analyze data to craft policy, monitor employee safety, and, remarkably, recent Epidemic Intelligence Service (EIS) fellows (the early-career epidemiologists who often show up first when something alarming appears).
Now, nearly a week later, several of those “reductions in force” (RIFs) are reversed. The whiplash reinforces a dispiriting pattern: act first, think later. Call it the chaos formula: “flood the zone” and let the fallout be someone else’s problem, the media (both mainstream and social) scrambling to keep up.
A quick word on the euphemism “RIF.” These are not abstractions. These are human beings who chose public service and were told they were fired for no cause. Imagine opening that email after years of outbreak response. The last time I commented on the chaos at CDC, one of the readers articulated a view that I 100% share:
My interactions with the rank and file members of the CDC when I have reached out during Ebola, mpox, and individual patients with rare parasitic infectious diseases showed their wide-open hearts and their knowledge – they cared so much for people they would never meet. My heart aches for this loss, and our patients will be worse for it.
In that post, I further argued that anger over COVID-19 policies fueled these attacks on the CDC. Some of this site’s loyal readers disagreed, saying this would have happened anyway. Their view: Certain quarters harbor such hostility toward government actions — and especially public health policies — that they now dismantle the CDC just because they can.
In support of my theory, however, a physician I know — one of our former trainees here in Boston — shared a strongly worded critique that began, “With all due respect…” (Never a comforting way to begin.) He viewed the CDC’s pandemic response as a “Chernobyl-level catastrophe,” citing examples such as school closures, toddler masking, the six-foot rule, and universal booster mandates. His plea was for the agency to acknowledge its mistakes and rebuild trust.
Not long after, I received a note from a government official who, while less fiery in tone, made a similar point. He wrote that we in the infectious diseases and public health communities underestimated how angry many Americans felt about being told to follow rules that, to them, never made sense — especially after the first year of the pandemic. He, too, cited the same examples, with a particular emphasis on school closures and rules about childhood masking, both acting as flash points that eroded public confidence and frustrated even medically sophisticated people.
Here’s my view: Two things can be true at once. Some policies were eventually proven to be flawed and dismantling the country’s premier disease-control agency is a terrible form of self-harm. Regarding the former: In the context of the tremendous unknowns of dealing with a pathogen never before encountered on this planet, could we expect perfection on policies and guidance from any individual or any group?
And were the missteps so grave that we should now systematically gut the institution that in recent history led the responses to Zika, Ebola, and the 2009 H1N1 pandemic? I don’t remember anyone complaining then. If avian influenza accelerates, who coordinates a national response? Do we really want measles to become re-established as a childhood illness in this country without anyone keeping track of outbreaks and trying to intervene? It looks like dengue and Chagas disease are about to take hold here — is anyone watching that? What’s going to happen the next time we need to consult someone with a rare parasitic disease?
We can debate pandemic guidance (please do, for transparency’s sake), past and future. But if we keep cutting the CDC off at the knees, someday there may be nothing left to reform, the damage will be irremediable.
And what then? Rebuild, slowly, during the next public health emergency? Sounds like a recipe for disaster.
Personal note. In other sad news, our dog Louie died this week. We’re heartbroken and grateful for the kind messages so many of you sent. Here’s more information about what happened, plus a remarkable video.
October 10th, 2025
DOTS: Optimism Around a “Negative” Dalbavancin Trial
 The DOTS randomized clinical trial of dalbavancin versus standard-of-care for Staph aureus bacteremia (SAB) just landed in JAMA, where it undoubtedly will be featured in numerous ID, hospitalist, and medical resident journal clubs over the next several months. Proof: one of our great second-year ID fellows tagged it immediately for his journal club literally the day it was published.
The DOTS randomized clinical trial of dalbavancin versus standard-of-care for Staph aureus bacteremia (SAB) just landed in JAMA, where it undoubtedly will be featured in numerous ID, hospitalist, and medical resident journal clubs over the next several months. Proof: one of our great second-year ID fellows tagged it immediately for his journal club literally the day it was published.
Led by Drs. Nicholas Turner, Thomas Holland, and a seasoned group of clinical investigators, it didn’t hit superiority on the primary endpoint, but clinical efficacy matched standard therapy.
Here’s why I’m optimistic despite this “negative” study:
- The primary outcome (Desirability of Outcome Ranking at day 70, DOOR) favored dalbavancin 47.7% of the time. That wasn’t statistically superior to standard care, and explains the cautious tone in the paper. But DOOR mixes clinical response, complications, safety, mortality, and quality of life; failing superiority there is not the same as failing clinically.
- Clinical efficacy? Essentially equal. On the prespecified noninferiority secondary endpoint, clinical efficacy at day 70 was 73/100 with dalbavancin versus 72/100 with standard therapy (difference 1.0%, 95% CI −11.5 to 13.5). When you focus on the question “Did the patient get better?”, dalbavancin did fine.
- Think of the comparison — two 1500-mg doses of dalbavancin (days 1 and 8) versus weeks of daily IV therapy. If outcomes are similar and safety is acceptable, this sort of study is exactly how indications and comfort grow for long-acting drugs. No peripherally inserted central catheter (PICC) line! No enrollment in outpatient parenteral antimicrobial therapy (OPAT)! No need for home delivery and storage of medications! No vancomycin levels! Hooray! The accompanying JAMA editorial, by Erin McCreary, PharmD and Dr. Preeti Malani, strikes a similar optimistic tone. Based on many years of hearing patients complain about PICC lines and OPAT, I’m also assuming that the quality of life instrument did not capture the specific tortures of home OPAT we all know and have grown to hate.
- Who participated really matters. Importantly, patients with left-sided endocarditis, CNS infection, retained infected prosthetic material, or severe immunocompromise were excluded. So although “uncomplicated” SAB was excluded, this wasn’t the everything-and-the-kitchen-sink group either — more like “intermediate complicated,” including right-sided endocarditis and other complicated bacteremias after initial clearance.
The study isn’t perfect, of course, with limitations acknowledged by the authors, but importantly none of them negate the findings. It was open-label, but I’d argue by necessity — the standard-of-care regimens were diverse, and exposing someone to the risks of a PICC line for weeks just for study purposes can’t be justified. Also, the benefits of two infusions would be greatly diluted if everyone had to get daily IVs. With only 200 participants, DOTS was relatively small, with enrollment taking over 2 years despite being open at 23 sites — perhaps because it was hard for investigators to thread the needle on the complicated-but-not-too complicated SAB inclusion criterion. Finally, at least some staph bacteremia is now managed with oral therapy, in particular after clinical stability is achieved with IV; this was not part of the comparison in this study.
(I added that one for you, Dr. Brad Spellberg. You’re welcome.)
OK, elephant in room time: The infrequent use of dalbavancin for inpatients currently is mostly about money, not microbiology. It’s no secret that if dalbavancin weren’t so eye-wateringly expensive, and if inpatient reimbursement weren’t DRG-based, we’d be using it a whole lot more already.
Price estimates hover in the multi-thousand-dollar range per 500-mg vial or per 1500-mg dose, which is painful for DRG-paid hospital stays even if it saves nursing time and averts line complications later. Who wants to be responsible for a pharmacy budget that includes several thousand dollars for a single antibiotic dose? I reached out to the lead study authors; an economic analysis is in progress.
But let’s back up and take a broader view. We recently heard a talk from Dr. Priya Nori about their experience broadening the use of dalbavancin in their healthcare system, with endorsement from their C-suite. They have found that dalbavancin can be a safe OPAT workaround for patients with barriers to long-term IV access, sometimes enabling earlier discharge and fewer complications, both of which substantially offset the cost (especially the early discharges). The fact that the drug is extremely well tolerated gives additional support for its more widespread use.
So yes, DOTS was “negative” on its primary outcome. But it also showed that dalbavancin performs about as well as what we do now, with far less infrastructure: no PICC, no daily infusions, no vancomycin levels, fewer moving parts. That alone should make the economics work, at least in healthcare systems looking at the global aspects of care rather than just an inpatient antimicrobial pharmacy budget.
Interested in what readers think — will these trial results increase use of dalbavancin for Staph aureus bacteremia? Have at it in the comments.
October 3rd, 2025
Update on Louie and One Interesting ID Fact

First, I want to express my sincere thanks to all those who commented, emailed, and texted about our dog Louie. My family and I truly appreciate the concern and the feelings people shared about their own dogs.
And how about these selected bits of wisdom, posted by Dr. Gordon Huth?
“Dogs’ lives are too short. Their only fault, really.”—Agnes Turnbull
“The world would be a nicer place if everyone had the ability to love as unconditionally as a dog.”―M.K. Clinton
“Fall in love with a dog, and in many ways you enter a new orbit, a universe that features not just new colors but new rituals, new rules, a new way of experiencing attachment.” ―Caroline Knapp
“The only creatures that are evolved enough to convey pure love are dogs and infants.”―Johnny Depp
“A dog has one aim in life … to bestow his heart.”―J.R. Ackerley
“Dogs have a way of finding the people who need them, and filling an emptiness we didn’t ever know we had.”―Thom Jones
Now, for the update and the background for why I wrote such an alarming post. (For those who tried to read it last week, and the link wouldn’t open, I posted it over here.)
After a very difficult morning walk last week, I took him urgently to the excellent, local animal hospital, Angell Memorial. Well, it was hardly a walk ― he could barely get over the curb, and I had to carry him home. Scary.
The kind veterinarian admitted him overnight, they did an ultrasound and cardiac ECHO, and the next day discharged him home with a terrible diagnosis: a suspected hemangiosarcoma, leading to a pericardial effusion and ascites, along with likely metastases in the lungs. Here ― take a look at the discharge paperwork (I’ve highlighted some of the low points):

It was quite clear that they did not expect him to live much longer; they also verbally advised us to arrange for home euthanasia within a week. Through sadness and tears, we scheduled it, and I shared the information on this blog because, as I wrote, it was therapeutic.
This has been such a wonderful community all these years, and it didn’t disappoint! Wow, I even heard from non-dog people ― including my mother and my first editor on this site, Mimi Breed. We know you don’t get the dog thing, but we appreciate the sympathy nonetheless.
But guess what? Louie didn’t understand the discharge instructions. Hey, he’s not so great with fancy medical terminology. Plus, he can’t read.
So after the anesthesia from his procedures wore off, he’s pretty much back to his baseline self ― a little less perky, perhaps, but still happy to see us, still eating, still wagging his tail. That’s a new picture of him up there at the top, after he scooted up the stairs. No more of those scary walks, at least not yet.
Look, we’re not in denial ― at least not completely. We’re medical people, after all. The professionals clearly gave him a grim prognosis, and we accept that; he won’t be with us for long. But to quote my wise colleague, social worker extraordinaire Susan Larrabee, you know your dog’s time has come when “your dog can no longer do dog things.” Louie can still do dog things. And that’s good enough for us. Euthanasia on hold, at least for now.
And since this is an ID blog, here’s a closing fact: we literally carry pieces of our dogs with us. Studies show that people and their dogs exchange microbes through shared living space and touch, and that the dog gut microbiome is astonishingly similar to our own ― it’s much closer to humans than to pigs or mice. The study used a microbiome gene catalog containing 1,247,405 genes, which sounds like plenty to me.
So when someone says that a dog becomes part of the family, it’s not just sentiment ― it’s science.
(h/t to Paul E Terrill for sharing this song.)
September 26th, 2025
My Dog Louie, the Best Dog Ever, Is Seriously Ill

We got some bad news about our dog Louie, the world’s greatest dog. Writing his story here is therapeutic, so forgive the oversharing.
In January, 2013, my wife forwarded me this email:
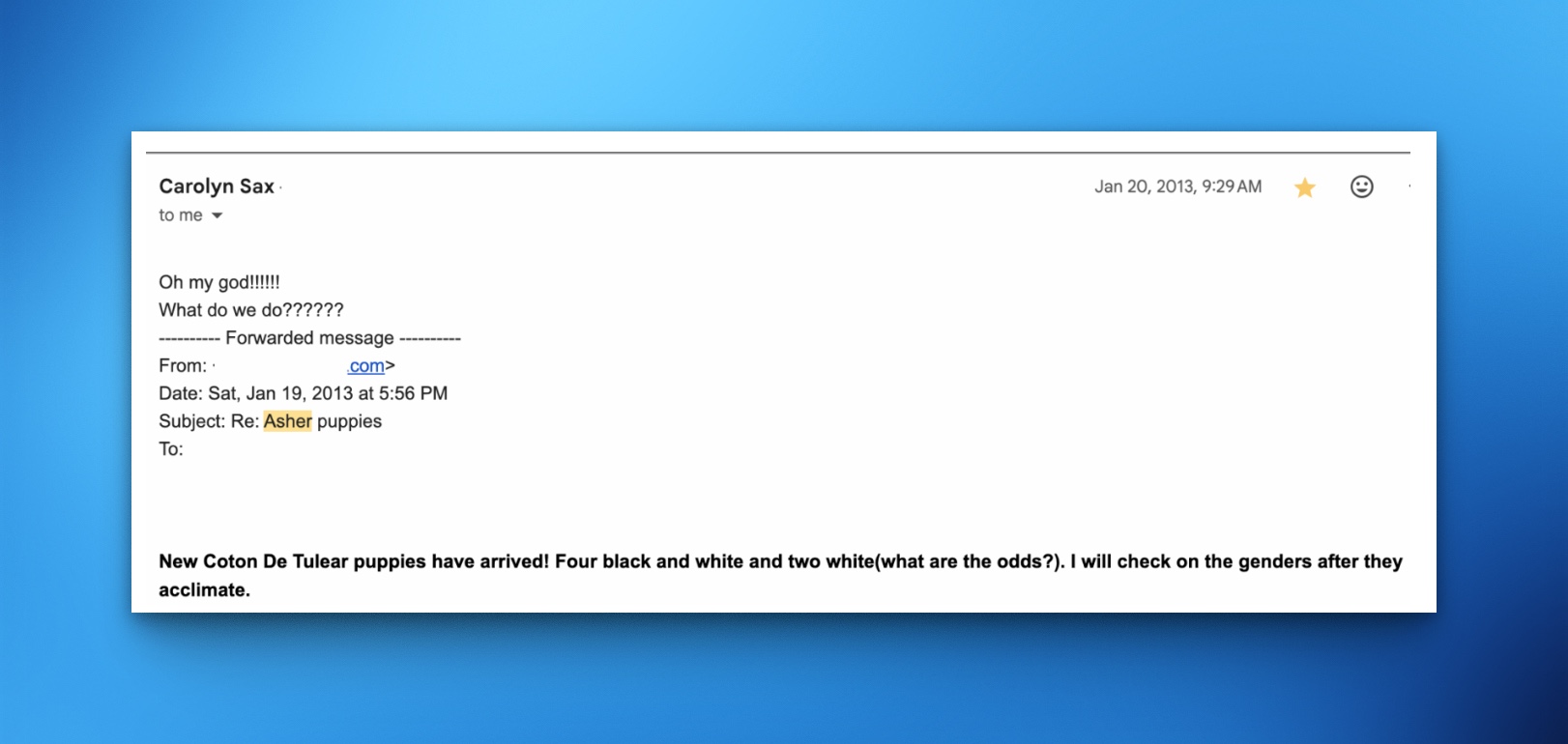
She doesn’t usually use 5 exclamation points or 6 question marks, and the unusual punctuation betrayed her excitement. The answer to “What do we do??????”, of course, was to claim one of these puppies; this would be her first dog.
The father of these pups — Asher was his name — was a special dog, a little guy who reigned supreme at the local gift shop, Wild Goose Chase. He greeted visitors with kindness, bright eyes, and a wagging tail, easily claiming his title as The World’s Cutest Dog. His owners even printed up his own card, one my wife kept in her wallet just for this opportunity.
A couple of months later, after Asher’s pups had grown up a bit, we met the very tired mom and the whole gang of her offspring at their home in western Massachusetts. We chose our favorite — a spotted little guy we called Louie, and brought him home to join our family.

Love at first sight.
Louie was no purebred, as the black spots gave away the unfancy part of his genetic lineage. Embark (yes, we checked) told us he was half Coton du Tulear, and half “super mutt,” a term I particularly like. Since this was always cumbersome to explain, when others asked what kind of dog he was, I often just said he was a mix, or a Havanese, since that’s what he looked like and “Havanese” was easier to say.
Over the years, we’ve bonded with Louie in a way that only dog lovers can understand — it’s nothing unusual, but it’s magic nonetheless. Here’s what happened:
- He immediately learned his name, and responded to it instantly. Of course his name is Louie.
- He excelled at eye contact, melting your heart. Stare your way to bigger meals.
- His sleep cycle gradually fell in sync with ours.
- We learned his loves and hates, based solely on the barking and yipping sounds he’d make at the door when he spotted a favorite (the mailperson who always brought him a Pup-peroni) or an enemy (a black lab that somehow embodied pure evil to him).
- His tail and bright eyes gave away excitement for every adventure (We’re going to the park!) or incoming snack (Would you like a treat?).
- Most importantly: He exuded love and adoration for us with zero ambivalence, expressing it regularly without ever, for a moment, getting tired of our company.
The years of love flowing from him made us utterly smitten. Someone came to my office recently, and noted that though the pictures of my family outnumbered the pictures of Louie, the biggest one in size was the painting my son’s friend made of our doggie, in exchange for a contribution we made to an animal services organization. Guilty as charged.
And yes, I’ve featured him here on this site, probably too much, and probably to the annoyance of those who just don’t get the dog thing. A sampling:
- There was a post about dog-related infections used as an excuse to post pictures about cute dogs, including Louie.
- How about this one about the importance of eye contact in clinical medicine? A perfect chance to show-off Louie’s super skill.
- Or another one, about the time he was attacked. He recovered quickly and harbored no hard feelings, what a good dog.
- I featured him with my brother’s and sister’s dogs, Zelda and Zoe. Why not?
- Here he posed with his favorite dessert plates, the china picked up by my wife in the bargain bin at local store. Yes, we bought a full set.

So lots of Louie here, the Star of the Show on this ID blog.
When I threw his toy or a ball, and he chased after it, he came romping back in a way perfectly embodied by the Charles Barsotti cartoon with the caption, And the crowd roars!
Wow, he learned to wag his tail in exchange for a treat! What a genius! He can even ride a bike!
To the non-animal lovers, I understand this post might not be your cup of tea. This kind of relationship is not for everyone, nor does it have much logic behind it. Why do we grow to love these animals so much? What causes us to generate endorphins when we pet them and stare into their eyes? Why do some people cry more at the death of their dogs than their family members? Makes zero sense.
We thought we had many more years of these tricks, and eye contact, and tail wags ahead.
But for those of us who have experienced it — this deep love for our pets that is returned by them more than a thousand-fold in exchange — I can assure you that watching them get seriously ill is utterly heartbreaking.
Which is why I’m writing this with tears streaming down my cheeks.
Let’s hope you rally, Louie, and that this is just some minor thing from a random scrap you picked up off the street, or a transient viral illness that just gets better with time.
But I am not optimistic. The unspoken messages conveyed by the vet as she told us of the ultrasound findings (we’re doctors, after all), and that she insisted he be admitted to the animal hospital for further workup — these do not engender hope.
I miss you already. ♥
September 19th, 2025
Two Drugs, Not Three: The DOLCE Study in Advanced HIV Disease
 Three-drug therapy has been the standard of care for HIV therapy for so long it’s difficult to shake the view that it must be more effective than two drugs. This is particularly the case for those with advanced, HIV-related immunosuppression or very high viral loads, as they provide an important stress test for all regimens.
Three-drug therapy has been the standard of care for HIV therapy for so long it’s difficult to shake the view that it must be more effective than two drugs. This is particularly the case for those with advanced, HIV-related immunosuppression or very high viral loads, as they provide an important stress test for all regimens.
Remember the pivotal GEMINI studies, comparing initial two-drug treatment with DTG/3TC with three-drug regimens of TDF/FTC plus DTG? Overall, they found that the two-drug arm was noninferior in virologic response. But because of concerns about inadequate activity of using fewer drugs, the trial excluded participants with HIV RNA >500,000. Plus, in the small number of those who started with CD4 <200, the results hinted at a suboptimal response in the DTG/3TC arm.
Now, along comes the DOLCE study, which had an entry criterion that required a CD4 <200, limiting the study population to people with advanced HIV disease — a bold move. Conducted in Argentina and Brazil, DOLCE enrolled treatment-naive participants who then were randomized 2:1 to receive open-label DTG/3TC (given as a single pill) or TDF/XTC plus dolutegravir (DTG). Concomitant chronic hepatitis B was exclusionary.
If you’re new to this HIV treatment business, allow me this geeky digression: “XTC” here doesn’t refer to the 1980s New Wave band, but to lamivudine (3TC) or emtricitabine (FTC), which are interchangeable from a safety and virologic perspective. The names come from their chemical structures — 3TC from one of the 3’s in 2′,3′-dideoxy-3′-thiacytidine, FTC from its fluoro group. And yes, confusingly, lamivudine is “L” in TLD, the abbreviation for tenofovir-lamivudine-dolutegravir. Got it? There will be a quiz.
Ok, back to the study. DOLCE enrolled 229 participants, 152 assigned to DTG/3TC, and 77 to TDF/XTC + DTG. At baseline, the median CD4 cell count was 116, and the viral load was 151,000. Fully a third of the participants would not have qualified for the GEMINI trials, as 23% had viral loads >500,000, and 10% greater than 1 million.
At week 48, 82% in the DTG/3TC arm and 81% in the triple-therapy group achieved viral suppression (<50 copies/mL). Time to virologic suppression and CD4 outcomes were similar, with no hint that three drugs performed better. Notably, responses remained comparable with both strategies even in the group with very high viral loads (>500,000). No treatment-emergent resistance occurred.
All studies have limitations, and this one is no exception — each acknowledged by the authors. The DOLCE trial was relatively small and not powered in its primary analysis to make specific claims about noninferiority (though a post-hoc analysis suggested it would meet standard noninferiority criteria). Plus, as an open-label trial, the DTG/3TC arm had the benefit of a single-pill formulation, which was not the case in the three-drug arm. With only two South American countries participating and the study being predominantly men, the results might not be applicable in other settings, though there is no obvious reason to doubt effectiveness in broader populations.
Limitations notwithstanding, credit to the investigators for filling this gap in knowledge about DTG/3TC with a challenging patient population. The astute (and always quite witty) Dr. Laura Waters wrote a fine accompanying editorial, appropriately stating that the data are reassuring from the virologic and safety perspective — and noting that the underperformance of DTG/3TC in GEMINI was likely unrelated to its antiviral activity.
But she also noted that still missing is a clear benefit of two drugs over a regimen that contains TAF (not TDF)/FTC plus either DTG or BIC — none of the studies to date have found evidence that “less is more” when it comes to this comparison, which was not done here. It may turn out that the true advantage of the three-drug regimens with DTG or BIC is not that they’re three drugs with greater anti-HIV activity — but that they contain tenofovir, which has a happy additional effect of being a highly effective treatment and preventive strategy for hepatitis B.
Now take it away, XTC! And this time I don’t mean 2′,3′-dideoxy-3′-thiacytidine.
September 10th, 2025
When Required Learning Modules Surprise You (In a Good Way)
 Like most clinicians, I have a checkered history with required online learning modules. You know the drill: click play, get lectured in monotone about hand hygiene or fire safety, then spend the next 8 minutes desperately searching for the fast-forward button, hoping it’s not disabled.
Like most clinicians, I have a checkered history with required online learning modules. You know the drill: click play, get lectured in monotone about hand hygiene or fire safety, then spend the next 8 minutes desperately searching for the fast-forward button, hoping it’s not disabled.
My personal low point (or high point, depending on your perspective) came years ago, when I proudly earned my Certificate of Completion for Disinfectant Wipes — Inpatient Settings. I even tweeted about it at the time, suggesting the Nobel Prize committee might want to give me a call.
(I’ve done you the favor of uploading proof to this site as the featured image, in case you doubt my credentials and are allergic to social media.)
So, when our hospital system announced an incentive program that required completion of something called the Equity-Informed High Reliability Organization (EIHRO) Level 1 Module, I wondered — who comes up with these titles? Is it a team of consultants paid by the syllable? My expectations hovered somewhere between “this is going to be tedious” and “could they imagine anything more soul-crushing?”
But here’s the shocker: it was excellent.
It’s not that the message was so profound or novel. It was the presentation — dynamic, engaging, and useful. Kudos to the narrator for keeping the topic moving along at a brisk pace and the video producer for including images and videos that didn’t look like they were pasted from a royalty-free site or bargain basement clip-art. The content came from a company called SG Collaborative Solutions, which was acquired years ago by a much more familiar player in this usually grim space, HealthStream.
The topic was how to address risk. Instead of the usual “don’t do this, do that” checklist, or calling bad events “mistakes” that trigger new policies, we need to understand the settings and systems that lead to adverse outcomes. It’s not enough to slap “Safety First!” into a mission statement. If you don’t understand the pressures of the actual workplace, the slogan does nothing. The recommended approach instead acknowledges that we all have competing priorities — productivity, efficiency, personal lives — and aims to optimize care in that realistic context.
Call me convinced. It’s a good lesson whether you’re a clinician caring for patients or at home reaching for a dish on a shelf that is a bit too high to reach comfortably. Wouldn’t it be smarter to keep a step stool handy than to wobble precariously on a kitchen chair? And for people who text and drive, just stop already. Which reminds me — to the cab driver in Philadelphia who was texting our entire drive to the airport, it’s not safer just because (as you confidently said when I asked you to stop), “It’s just my son.”
Now, if you’ll excuse me, I’m going to update my CV:
- 2019: Certified in Disinfectant Wipes
- 2025: Equity Informed High Reliability Organization (EIHRO) Level 1 Module
Could this addition tip the scales for the judges in Stockholm?
September 4th, 2025
End-of-Summer Musings — Hepatitis B, Dalbavancin, Alpha-Gal, and More
 The last time I did one of these quick “musings” posts, I listed 21, and someone asked me, “Why 21?” The answer — obviously — is that I originally planned on writing 20, but then had to add a 21st, just because that’s exactly how many points you need to win a ping pong game.
The last time I did one of these quick “musings” posts, I listed 21, and someone asked me, “Why 21?” The answer — obviously — is that I originally planned on writing 20, but then had to add a 21st, just because that’s exactly how many points you need to win a ping pong game.
So here are a bunch more, in honor of another important racquet sport, tennis, which this weekend will reach the culmination of its grueling season in the finals of the US Open. Stick around to the end to see how I tie it in.
1. Hepatitis B transmission occurred in a nursing home from a contaminated glucometer. As is commonly done, glucometers at the skilled nursing facility were shared among several residents. Even though the disinfection protocols were apparently followed appropriately, a person with chronic hepatitis B was the source of a transmission. In an era of near-universal hepatitis B immunization and dropping prevalence of chronic HBV, it’s worth remembering that among our blood-borne pathogens (HIV, HCV, HBV), HBV is by far the most contagious.
2. A strategy of two doses of dalbavancin 1 week apart for Staph aureus bacteremia was noninferior to 4-8 weeks of daily therapy. However, it was not superior based on the primary “desirability of outcome ranking,” or DOOR endpoint. I think noninferior for clinical endpoints is still important for something as transformative as just two doses, though cost remains a huge barrier for dalbavancin. And get ready for a bunch of studies that use this novel DOOR composite endpoint, which incorporates clinical success, infectious complications, safety complications, mortality, and health-related quality of life.
3. Alpha-gal syndrome is tick-related, is increasingly common — but no, it’s not an infectious disease. Raise your hand if, as an ID doctor, you have been asked anyway to comment on the diagnosis or management of this allergic reaction to the sugar molecule galactose-alpha-1,3-galactose. Here’s the chemical structure, in case you’d like to synthesize it during your free time.
4. I thought you couldn’t put the toothpaste back in the tube. Tell me — who would buy this gizmo? Even for only 10 dollars, I just can’t see the use case.
5. Two-drug initial therapy with dolutegravir/lamivudine provided similar results to a standard three-drug regimen of TDF/FTC plus dolutegravir, even in people with advanced HIV disease. I’ll have more to say about this important study in a later post, along with the fine editorial by Dr. Laura Waters — who also issued some wise career advice as she is about to leave her current position in Britain’s National Health Service.
6. China this summer battled an increase in chikungunya cases in Guangdong Province. We periodically see cases in the United States in returning travelers, especially coming from the Caribbean and South America. It is impressive how debilitating the post-infectious arthritis can be with this dengue- and zika-like virus.
7. I received a bunch of supportive comments and emails about my rant against learning objectives, thank you. A favorite was one from Andrea Demeter, who cleverly took to AI to find reasons to get rid of them — which she must have known would prove my point! But if they’re here to stay, maybe in the future AI can generate the learning objectives, generate the lecture and PowerPoint slides, and then generate the evaluation of whether the lecture met the learning objectives. Magic! And no learning required.
8. Federal, state, and professional societies now issue competing (and sometimes contradictory) vaccine recommendations. Examples: Florida plans to eliminate school requirements for vaccines, while California, Oregon, and Washington band together to ensure communication of “credible information” on vaccines, and Massachusetts makes COVID boosters available for all older than 5. You’d think we live in different countries or something. My Boston ID colleague, Dr. Shira Doron, said it best: “The chaos just doesn’t end.”
9. This group argues persuasively that we should designate Chagas disease endemic in the United States. There’s no doubt that this parasitic infection is far more common in our country than we realize, and that autochthonous transmission occurs here — especially in the Southwest. The labeling of the United States as nonendemic leads to inadequate surveillance and clinical knowledge about this potentially serious parasitic infection.
10. Some of the “friends” that dogs bring home are more welcome than others:
11. In this randomized clinical trial conducted in adults older than 65, high-dose influenza vaccine did not prevent pneumonia or hospitalization better than standard dose. The study was large and well-conducted, enrolling 332,438 participants. 332,438! Is this study the final word on this strategy as we await a better flu vaccine?
12. Switching people with HIV on second-line boosted-PI regimens to BIC/FTC/TAF was noninferior to continued PI therapy. Results reinforce the results from the 2SD and VISEND (Arm A) studies, but add baseline retrospective proviral DNA resistance testing in the bictegravir arm, which demonstrated a high prevalence of NRTI resistance. Hat-tip to Drs. Patrice Severe and Serena Koenig and the GHESKIO team for completing this clinical trial during a time of extraordinary civil unrest in Haiti. (I was a co-investigator.) Good accompanying editorial.
13. There have been six rabies deaths in the US in the past 12 months. This is more than the usual number — typically one to three, some years there are zero — for reasons that are unclear. Can anyone find reference to this on the CDC site? Because tracking rabies and the previously mentioned Chagas disease are exactly why we need a strong agency to do national surveillance for infectious threats. The states simply can’t do it all.
14. If you like documentaries about brilliant songwriters, put these two on your list. The first is about Billy Joel — you might have heard of him (you think?); he has a fascinating biography to back up his impressive song catalogue. I enjoyed it a ton even though I’m lukewarm about much of his music. The more obscure offering is about Charles Fox, a person unknown to me before watching this film. But let’s just say if there’s a soundtrack for us boomers who watched TV growing up, he’s written it.
15. HHS cancelled nearly half a billion dollars in research support for mRNA vaccines. This follows cancellation of a grant to Moderna of 800 million dollars. Vaccine experts consider this technology the most efficient way to respond to a pandemic threat; it also has promise for immune-based cancer therapeutics. Discouraging.
16. I liked this critical piece that highlights issues with excess screening requirements in primary care. Problems include misplaced incentives, strategies that are hardly evidenced-based, and fatigued or distracted providers who then miss the important aspects of patient care. We saw this recently as our electronic medical record flagged absence of hepatitis B immunity in all patient’s charts — for what reason, exactly? Drove multiple clinicians nuts with frustration.
17. What’s going on with pivmecillinam? With gepotidacin? With sulopenem? All three have FDA approval to treat UTIs, none is yet available in US pharmacies. The full name of the last one is “sulopenem etzadroxil with probenecid,” but I defy you to type that out every time you write it.
Seventeen Musings! That’s the age of Serena Williams when she won her first tennis Grand Slam title in 1999. And now, in all it’s glory, is a piece that explains why you should play tennis if you can:
18. We fanatics all read and cheered this tribute to the world’s greatest sport. So much here to love: the growing popularity (pickleball notwithstanding), the fitness advantages, the effects on brain health, the chance to play a sport for a whole lifetime. For the record, I think there’s room in the world for lots of racquet sports — tennis, pickleball, padel, squash, racquetball, badminton, ping pong… And not that she needs it, but I’d bet this is the first time that the writing of Alexandra Moe has been featured in an ID blog.
Ok, eighteen Musings — that’s a good number. It even has its own Wikipedia page!
August 29th, 2025
Watching the Chaos at the CDC — with Sadness and Alarm

Image by Jackson Moccelin from Pixabay
Throughout my career as an infectious diseases doctor, the CDC has been a rock-solid source.
- Need reliable data on an outbreak? The CDC.
- Need thoughtful, evidence-based guidelines? The CDC.
- Need an authoritative reference for a consult question or to steer a colleague or trainee to the right place? The CDC.
- Need the latest, most accurate surveillance information on HIV or influenza or dengue or you-name-it? The CDC.
- Need advice for your friend doing anthropology research in rural Bolivia and wondering whether they should take malaria prophylaxis (yes), and what it should be (multiple options)? The CDC.
Did I always agree with every word? Of course not. Show me an ID doctor who always agrees with every guideline, and I’ll show you a cephalosporin that covers enterococcus.
For example, for years I’ve thought we’re too aggressive with “bat in bedroom, no bite” postexposure prophylaxis, preferring the Canadian approach. But I never doubted the people making the U.S. guidelines had done their homework with integrity; they weren’t part of some sinister rabies vaccine/immunoglobulin cabal. It was a difference in opinion, that’s all.
And I trusted the people. The ones I’ve known who have worked at the CDC are mission-driven and incredibly hard-working. They’re not looking for fame or fortune, to cultivate a brand, or to make a splash in the media. You don’t expect to see a TikTok video from a CDC official. They’re the kind of colleagues who disappear into data sets, surveillance reports, and fieldwork, all in the service of preventing disease and saving lives.
So what happened?
In a word: COVID. The pandemic was the public health earthquake of our lives, and the aftershocks continue. The CDC is one of its casualties. Instead of learning from missteps and strengthening the institution, we’re watching it be weakened — deliberately, in some cases, and it seems with pointless vengeance. The critics who scream the loudest treat it as if every imperfect COVID recommendation, every adjustment of guidance in real time, every policy now viewed as incorrect with 20-20 hindsight, was a betrayal and not a best effort to help us cope with this new, tricky virus.
Let’s acknowledge that perfection was impossible in the face of an unprecedented global crisis. Guidance had to evolve, sometimes rapidly. To punish the agency now is like telling tennis star Carlos Alcaraz he can’t compete in the U.S. Open because he lost in the Wimbledon finals.
Yes, the stakes were high during COVID, but nobody — NOBODY — got it 100% right.
The result? One of our most reliable public health institutions is being destroyed by non-experts in medicine and public health. People with disturbing beliefs about infectious diseases in general and vaccines in particular. And who knows what the next year or years will bring? Who will we turn to then?
For those of us who have leaned on the CDC our entire careers, watching its destruction at the hands of non-experts is not just disorienting — it’s heartbreaking, and makes me very, very sad.
August 22nd, 2025
On the Internet, Nobody Read My First Blog

Not the site’s banner, but you get the point. (AI generated.)
In 2007, during the boom in online blogging, and right before the economy crashed, a medical education company contacted me and three other ID doctors to write a blog about HIV. The pitch was irresistible — write something about HIV on a regular basis (they didn’t say what), and we’ll promote it widely. Oh, and you’ll get paid!
The lead from the company certainly conveyed enthusiasm. He made the inevitable “viral” pun (ha ha, we all chuckled politely), and unveiled a clever name. It had “expansion potential,” he said: We’ll start with this one on HIV, but later we could launch one on Oncology, Dermatology, Hirudotherapy*.
(*Leech therapy — so not really that.)
He also mentioned how much interest he’d already received from the various companies that developed HIV treatments. Indeed, they planned to sell ads once the internet traffic came pouring in. It was all very promising.
I enthusiastically signed up. Then I wrote a few pieces, none of them in hindsight very good, and sent them to the person responsible for posting them online.
It launched in the spring of 2007, including an inaugural piece of mine called “Some Non-Scientific Thoughts on CROI” where I complained about CROI holding its opening night during the Academy Awards — in Los Angeles, no less. (Yes, they did that.)
Then I waited for the flood of insightful comments. You know, Web 2.0.
The response?
It got worse from there. Reader engagement went from sparse to nonexistent. The site was glitchy, forcing people to reset their passwords for no apparent reason. You can only clear your browser cache so many times before bolting. Even in 2007, patience for that kind of frustration was in short supply.
And the design of the site? It typified the overall look of the early-ish internet — low resolution, bad colors, possibly done with an open-access graphics program by someone’s 8th grader. You want pop-up windows? We’ve got pop-up windows!
The combination of sporadic content and technical obstacles hardly created the kind of user experience to encourage participation. The site closed up shop in less than a year, a much less famous failure than Lehman Brothers, which shared a similar fate and termination date.
But despite the false start, I’m grateful to the organizers for their optimism and for getting me going — you have to start somewhere. That was the lesson I learned in hindsight. It won’t always be a success, but it’s worth putting the words on the page, even if you later cringe at the whole effort.
That’s true for any creative pursuit — painting, music, pottery, bonsai tree cultivation. False starts are still starts, cringeworthy though they may be.
Most importantly, when the NEJM Journal Watch editor Matt O’Rourke considered expanding the Journal Watch content to include blogs, I had some experience with the medium, and could send him some of my posts — now revised, reworked, and improved, of course!
(Revision is an amazing thing. Another lesson learned.)
Would I have been able to launch this effort without that initial experience? Unlikely.
And as a reminder that even the most enduring internet creations start somewhere, here’s Peter Steiner talking about his own legendary contribution to world humor — the brilliant, timeless cartoon “On the Internet, nobody knows you’re a dog.”
It started just another sketch in a pile of many, and was hardly his first try.

