An ongoing dialogue on HIV/AIDS, infectious diseases,
November 4th, 2010
XMRV and CFS: More Yay and Nay
 Does XMRV cause Chronic Fatigue Syndrome? Or more accurately, is it even associated with CFS?
Does XMRV cause Chronic Fatigue Syndrome? Or more accurately, is it even associated with CFS?
I’ve been putting off writing about this for a while, as I knew colleagues of mine had a paper in press on the topic, and I wanted the dust to settle a bit more on the controversy.
But of course the controversy, and the scientific inquiry, are just getting started. There was a recent HHS meeting on the topic; anecdotal reports of patients with CFS receiving antiretroviral agents for treatment; patients with the condition have been discouraged by one group from donating blood.
Now, my colleagues’ paper has been published in The Journal of Infectious Diseases — and it shows no detection of XMRV in patients with CFS, and furthermore no XMRV in comparison groups of patients with HIV, rheumatoid arthritis, transplantation, or a group just presenting for general medical care.
The score is getting hard to tally, especially because negative studies get way less press. On the plus side, there was the original Science paper that got the field started, and the more recent PNAS one; the latter, oddly, found a slightly different retrovirus (MLV).
On the minus side? In addition to the JID paper from Boston, there have been negative studies from the Netherlands and two from Britain (here and here).
I am sure there are many others in various stages of gestation.
How could highly-qualified scientists come to such disparate conclusions? Here are some admittedly unoriginal thoughts:
- CFS has many causes. (See here for my view.)
- Related: Some CFS is of the “epidemic” variety; sporadic cases are different, less likely to be caused by infection.
- Geographic variability in XMRV incidence/prevalence.
- Different assays and/or different primers used in different labs.
- Contamination of assays. (It is PCR, after all. Isn’t that an inherent risk for any highly-sensitive amplification procedure?)
- Replication of XMRV is variable, or low level, a la HTLV-1 or HIV in “elite controllers.”
- XMRV detection is an epiphenomenon, and “true-true, and unrelated” — hence detection of the virus may be a random event, or merely an association.
- Some other issue no one has thought of.
Regardless, I highly recommend the editorial by Mary Kearney and Frank Maldarelli that appears in the same issue. They call for the following:
- Standardization of detection assays.
- Prospective epidemiologic surveys.
- Sharing reagents and samples. [Amazing this hasn’t been done yet!]
- Comprehensive and rigorous phylogenetic sequence analysis.
- Development of tractable animal models.
In addition, they have strong words for those who are prescribing antiretrovirals for individuals with CFS:
At this time, such an approach is premature and medically indefensible outside the secure oversight of a well‐controlled clinical trial. “Real world” coping with severe diseases like chronic fatigue syndrome and prostate cancer creates understandable desperation on the part of patients, caregivers, and health care professionals. Such pressures are not justification for testing of therapies in an uncontrolled manner. Indeed, because they are of no help whatsoever to other patients, physicians, pharmaceutical companies, or regulatory agencies, such uncontrolled therapy works directly against the goal of providing effective therapy to the million or more individuals experiencing these serious conditions.
Wise words indeed.
October 29th, 2010
With HIV Medication Adherence, It’s Not a Competition
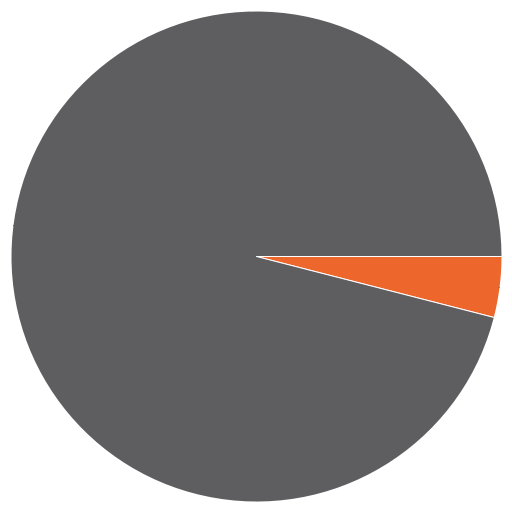 There has been an irresistable urge for people — doctors, public health officers, politicians, journalists, the usual pundits — to compare adherence to HIV treatment in resource-rich vs. resource-limited setting.
There has been an irresistable urge for people — doctors, public health officers, politicians, journalists, the usual pundits — to compare adherence to HIV treatment in resource-rich vs. resource-limited setting.
I suspect this is because the whole issue got off to a famously bad start in 2001, when then-head of the U.S. Agency for International Development (USAID) Andrew Natsios said in an interview with the Boston Globe that Africans:
… don’t know what Western time is. You have to take these (AIDS) drugs a certain number of hours each day, or they don’t work. Many people in Africa have never seen a clock or a watch their entire lives. And if you say, one o’clock in the afternoon, they do not know what you are talking about. They know morning, they know noon, they know evening, they know the darkness at night.
Yikes.
The reality, of course, is that patients in Africa and other resource-limited settings have been just as adherent to HIV treatment as people in developed countries.
But are they more adherent?
Yes, says this article in the New York Times. And yes again, says this widely-quoted meta-analysis, published in JAMA in 2006.
But I don’t buy it — never have.
In fact, in his otherwise inspiring talks on how to bring lifesaving health care to poor countries, my doppelganger Paul Farmer has a small section I absolutely hate. (Am I allowed to criticize Paul Farmer? Sure — he knows it.) It’s where he compares adherence rates for Haitians in his community-based programs to that of patients discharged from the inpatient medical service at Grady Memorial Hospital in Atlanta.
Not surprisingly, the Haitians do better. But that’s not really a fair comparison, because in Haiti the people on treatment are actively seeking care, while with these particular patients at Grady, they are actively running away from it until they get so sick they need to be hospitalized.
At the great risk of oversimplifying the issue — and acknowledging up front that I do my work exclusively here in the USA (but in a setting with a very broad range of patients) — here’s my take:
- Most people with HIV — everywhere — are phenomenally adherent to therapy. Not only that, it gets better over time. Excellent adherence is especially common in those who, as mentioned above, are seeking care rather than trying to evade it. Remember the AZT-every-4-hours group? The indinavir-every-8-hours-on-an-empty-stomach group? With today’s easier regimens, lots of patients barely ever skip a dose, and they look at you like you’re crazy when you ask them how often they miss. And Carlos del Rio tells me that many of them are receiving care right near Grady at the outstanding Ponce de Leon Center.
- It is incorrect to think that this ability to take medications differs in different countries. In the same way that Natsios got it wrong about Africa, it’s wrong to assume that we can’t do it here based on antiquated data, examination of only a selected subset of patients, or reverse cultural assumptions. I heard someone once say at a lecture that adherence was worse in the United States than in Kenya because US HIV patients “take everything for granted.” Yikes again.
- The huge benefits of HIV therapy notwithstanding, some people just don’t get it, and they drive us crazy. Everyone knows a small group of patients — see pie graph above, as my unscientific estimate puts it at 5-15% — who just won’t play the ART game by the rules, or at least won’t do it consistently. They are in denial; or they don’t really believe you that HIV is lethal; or they have side effects to everything; or their life is in total chaos; or they have psychiatric illness; or they have drug or alcohol problems. Or all of the above. And you know what? Though small in number, such people are everywhere (even in Africa), and found with every disease. They occupy a tremendous amount of our clinical energies and a disproportionate share of resources — and not surprisingly, it’s this group that gets hospitalized repeatedly at Grady, as well as every other hospital that sees its fair share of HIV patients.
So the next time you hear someone make a broad generalization about adherence being better in City A vs B, or Country X vs Y, remind them that people are people — and adherence to HIV therapy is likely to be the same everywhere.
That is, excellent until proven otherwise.
October 22nd, 2010
How to Figure Out the Length of Antibiotic Therapy
 One thing we ID doctors know — that other clinicians simply don’t — is how long to treat a patient with antibiotics.
One thing we ID doctors know — that other clinicians simply don’t — is how long to treat a patient with antibiotics.
I was reminded of this special power by these recent events:
- An excellent fellow from the hospital’s Critical Care program rotated through our division recently. When asked about what she wanted learn from the elective, the first thing she said was figuring out — finally — how long an antibiotic course should be.
- We received a consultation on an unfortunate case that had been hospitalized since approximately the time of the Gerald Ford administration. You know the type — multiple surgeries gone bad, several GI anastomotic leaks, innumerable tubes and drains, abundant highly-resistant bacteria and yeast cultured from each drain. The question: “How long should we treat the acinetobacter?”
- This great article in the New York Times by Harvard “Happiness” Professor Daniel Gilbert, who relays being given a 7-day course of antibiotics by a doctor. “I understood why I needed to complete the full course, of course. What I didn’t understand was why a full course took precisely seven days. Why not six, eight or nine and a half? Did the number seven correspond to some biological fact about the human digestive tract or the life cycle of bacteria?”
The answer, Dr. Gilbert, is that this is highly-specialized knowledge, rarefied information that only 100% Board-Certified, USDA-inspected Infectious Diseases Doctors know. And since I’m concerned that your article might give readers the wrong impression about our scientific credibility, I’ll now divulge what we’ve learned, and how to apply it.
To figure out how long antibiotics need to be given, use the following rules:
- Choose a multiple of 5 (fingers of the hand) or 7 (days of the week).
- Is it an outpatient problem that is relatively mild? If so, choose something less than 10 days. After application of our multiples rule, this should be 5 or 7 days.
- Is it really mild, so much so that antibiotics probably aren’t needed at all but clinician or patient are insistent? Break the 5/7 rule and go with 3 days. Ditto uncomplicated cystitis in young women.
- Is it a serious problem that occurs in the hospital or could end up leading to hospitalization? With the exception of community-acquired pneumonia (5 or 7 days), 10 days is the minimum.
- Patient not doing better at the end of some course of therapy? Extend treatment, again using a multiple of 5 or 7 days.
- Does the infection involve a bone or a heart valve? Four weeks (28 days) at least, often 6 weeks (42 days). Note that 5 weeks (35 days) is not an option — here the 5’s and 7’s cancel each other out, and chaos ensues.
- The following lengths of therapy are inherently weird, and should generally be avoided: 2, 4, 6, 8, 9, 11, 12, 13 days. Also, 3.14159265 days.
In this highly data-driven exercise, it is imporant also to note the number of rules — seven, as in days of the week.
That did not occur by chance.
October 12th, 2010
Worlds Collide: Roberto Alomar and HIV
 Now that a second woman has accused Roberto Alomar of having HIV, it’s probably time to give this sad story a look-over. After all, how often do these two worlds of mine — HIV/ID (work) and baseball (lifetime hobby — my wife would say that’s a collosal understatement) actually meet?
Now that a second woman has accused Roberto Alomar of having HIV, it’s probably time to give this sad story a look-over. After all, how often do these two worlds of mine — HIV/ID (work) and baseball (lifetime hobby — my wife would say that’s a collosal understatement) actually meet?
For those unfamiliar with the basics: Alomar was a staggeringly good player, in the majors from 1988-2004, winning 10 Gold Glove Awards and making the All Star team 12 times. He very nearly was elected this year to the Hall of Fame in his first year of eligibility (a very big deal), and all the baseball cognescenti think he’s an eventual shoe-in since he’s one of the best second basemen in history.
That’s the good part.
On the other hand, there was this strange spitting incident. And then, in 2009, an ex-girlfriend filed a $15 million lawsuit saying he lied about his HIV status; Alomar denied the charges (sort of), and was defended by his then girlfriend — who later became his wife. Then last week, the wife sued Alomar with similar allegations.
I can’t get into the “she-said, he-said” part, as I have no information beyond what’s available in the press. But if we start with the premise that Alomar might have HIV, here are some general thoughts:
- Any reason why someone with HIV couldn’t play baseball? I can’t think of one.
- Could there be anything further from Magic Johnson’s 1991 disclosure than these sordid legal proceedings? As I recall, Johnson’s wife was extraordinarily supportive, at least as relayed to the public. Then he used his HIV diagnosis as a way to encourage people to find out their status, to protect others from getting infected, and to take advantage of treatment — an incredible example of turning something terrifying and stigmatized (especially in the early 1990s) into something good. One of John Bartlett’s favorite comments is that hepatitis C needs a Magic Johnson to make its case. (No, Evel Knievel, may he R.I.P, does not count.)
- Related to the above, you can be sure society’s reaction to Magic Johnson’s disclosure would have been different if he’d acquired HIV from injection drug use or sex with men. (Read the section “Time to Move On” here for how this issue has been reported.) One of the painful things about HIV is the tendency for many to lump cases into those perceived as “innocent” vs “he/she deserved it.”
- For another stark example of how HIV differs from other serious diseases (even those related to unhealthy choices), Hall of Famer Tony Gwynn has just been diagnosed with salivary gland cancer, likely due to chewing tobacco. The response? Mostly sympathy, very little blame or snark.
- Last, here’s a great graphic on how major league baseball players compare to the rest of society. Pretty much says it all.
My view? I hope Alomar gets elected to the Hall of Fame next year — from the baseball perspective he definitely deserves it.
And if he does have HIV, wouldn’t that be something if he acknowledged it during his acceptance speech in Cooperstown.
October 7th, 2010
Post-Halladay Video Treat: Prospects for HIV Cure
 For a little entertainment between playoff games — but how could anyone beat the guy in the picture? — you might want to check out this interview I did with Dan Kuritzkes about the prospects for an HIV cure.
For a little entertainment between playoff games — but how could anyone beat the guy in the picture? — you might want to check out this interview I did with Dan Kuritzkes about the prospects for an HIV cure.
So which do you think we’ll see first — an HIV cure or a vaccine? And I don’t mean an isolated (and impractical to carry out on a large scale) strategy like the famous bone marrow transplant case, or the weak protective effect from the first “effective” vaccine study done in Thailand.
No, for this to count, it has to be a strategy for HIV cure that could be widely implemented, or a vaccine that is both highly protective and practical to distribute.
Around 7 minutes into the video, Dan lets us know his opinion!
October 1st, 2010
Five Friday Fasciolas
 As we await either the start of the baseball playoffs (or Spring Training), here is some ID/HIV content to consider, in no particular order:
As we await either the start of the baseball playoffs (or Spring Training), here is some ID/HIV content to consider, in no particular order:
- Could adenovirus infection be the cause of obesity? That would be the media take, especially from this highly-esteemed research journal, The New York Daily News. (Warning: kind of ugly photo in link.) But if you read the actual paper, the scientists appropriately note that the study just demonstrated an association with childhood obesity, not causation. Still — did you know that animal models show that adenovirus 36 infection increases body fat? I did not.
- Remember ceftobiprole? The “Fifth Generation” anti-MRSA cephalosporin approved for use in Europe, Canada, Hong Kong, etc and heading to the USA? Well you can take Canada, Switzerland and soon several other countries (Russia, Ukraine and Azerbaijan) off that list, as the drug is being pulled due to concerns raised by the FDA in their review. By contrast, it does look like ceftaroline (reminds me of Caroline, less so Carolyn) is on the way. My reason for optimism this time is the uninimously favorable vote from an FDA Advisory Committee meeting; here are the slides from the meeting last month.
- This MMWR paper on the prevalence of HIV among gay men in the USA got lots of attention, and appropriately so. The reason? “New CDC analysis finds 1 in 5 gay and bisexual men in a study of 21 major U.S. cities is infected with HIV, and nearly half (44 percent) are unaware; young men and men of color are least likely to know their status.” Important take-home message: In the effort to expand general HIV screening strategies, we can’t forget that there’s a critical role for targeted testing as well. Some would argue this is more important.
- One of the most widely-cited papers in all of HIV medicine in the late 1990s/early 2000s was this one, which showed that you needed to take 95% of HIV medications for the best shot at suppressing the virus. (I must have some fifty copies of this study in various slide sets scattered around my hard drive.) We’ve known for some time that the adherence bar isn’t set this high with today’s better regimens, but could one ever imagine that someone could take as little as 40% of their meds without much risk of viral rebound, provided it’s been preceded by long term virologic suppression? Not that I’m suggesting this as an actual strategy, but this should be quite reassuring for our successfully treated patients who think they can never miss a dose.
- Easily one of the un-funniest pranks I’ve heard about, maybe ever. Oh, what will kids think of next.
Enjoy your watercress salad!
September 27th, 2010
CROI 2011: February 27-March 2, Boston
 Yes, I know it’s already listed on the official web site.
Yes, I know it’s already listed on the official web site.
But since it’s in Boston, and since this post drove tons of internet traffic this way, why not cite it again?
And let’s all hope that February here in 2011 is nothing like February 1978.
Nah, that couldn’t happen …
September 17th, 2010
What Are These Conferences?
With ICAAC now completed — which took place in a city called Boston but seemed far, far, from home (see picture) — it seems timely to inquire about another form of “scientific” conference.
Every so often, I’ll receive an email like this (slightly edited to protect the sender, whomever he or she may be):
Dear Dr. Professor Sax,
On behalf of the Organizing Committee, I am pleased to invite you to attend the 1st Annual Global Conference on Infectious Disease Pharmacotherapy and Prevention, to be held in [insert economically booming city from Asia or the Middle East here] from 12-15 January, 2011.
We would like you to participate as our guest speaker, giving a keynote address on [insert topic here, likely only vaguely related to what I do] on a day of your choosing.
Already, Nobel laureate Dr. Griffin Doohickey [I made that name up] has agreed to chair the meeting. If you decide to participate, you will be joining several other internationally-recognized leaders in research who will be providing plenary lectures as well.
 I’ll admit that the first time I received one of these, I was extremely flattered — after all, who wouldn’t want to visit Dubai, or Beijing, or Taipei?
I’ll admit that the first time I received one of these, I was extremely flattered — after all, who wouldn’t want to visit Dubai, or Beijing, or Taipei?
But since none of these invitations actually offered to cover the expense of travel, accommodation, or registration, I became suspicious — could it be that most (if not all) of these meetings are bogus, designed to separate academics from their money by appealing to their professional narcisism?
If I’m right, I sure hope Dr. Griffin Doohickey hasn’t provided the organizers his credit card or bank account information. And if he has, there’s a Nigerian prince I’ve heard from who has a very promising investment opportunity.
September 6th, 2010
Treating Cellulitis: Getting the Answer Wrong and Right
 What’s the right antibiotic choice for cellulitis in the era of community-acquired MRSA?
What’s the right antibiotic choice for cellulitis in the era of community-acquired MRSA?
As astutely pointed out by Anne in her comment, the “correct” answer to the recertification question was #4, trimethoprim-sulfamethoxazole, for the following reason:
I am not a doctor, I am a test developer. Having exactly zero knowledge about the content here (could be Chinese to me), but based solely on the structural design of multiple-choice items, I would venture to guess that the correct answer (the “key” in testing parlance) is #4. I say this merely because it is significantly longer than the other three options (the “distractors”) and therefore leads one to believe that it contains the best information.
However, what does it mean that the person giving the correct answer isn’t a doctor, and admittedly has “zero knowledge” on the subject?
Well, it probably means that the question is flawed — which is obviously the case, since there’s no right answer.
Azithromycin is plainly wrong.
You migh choose dicloxacilln or cephalexin, but one over the other? And both don’t cover MRSA.
And trimethoprim-sulfamethoxazole doesn’t cover strep.
Don’t believe me? Read this recent paper on community acquired “nonculturable” cellulitis, which shows that a significant proportion are not surprisingly still due to beta strep, even in the era of community acquired MRSA.
In the real world of treating patients and not answering test questions, what we actually should do in this situation is hardly clear — diclox or cephalexin first then change if no response? TMP-SMX + amox?
I’d suggest that such ambiguous situations make for exam items that are more maddening than educational.
September 1st, 2010
Testing your Testing Skills
 Have I whined yet about how I’m part of the first Internal Medicine class that was not “grandfathered” through to eternal board certfication?
Have I whined yet about how I’m part of the first Internal Medicine class that was not “grandfathered” through to eternal board certfication?
If not, now I have.
So for you fellow test-takers, here’s another one for you, adapted somewhat from this delightful experience I’m required to go through every 10 years. Oddly, just like the last one reviewed here, it involves our old friend, MRSA.
18 year old kid scrapes his knee playing a sport, comes in with infection around injury. No purulence. Community MRSA is known to be in the community. What should you give him?
- azithromycin
- cephalexin
- dicloxacillin
- trimethoprim-sulfamethoxazole
Every so often one encounters a test question that is, frankly, just wrong. But in the often-bizarre world of “let’s pretend” patient care — a test question — you still have to put your money down.
So what’s the answer?

