An ongoing dialogue on HIV/AIDS, infectious diseases,
January 17th, 2013
Why the Results of the C Diff Study (You Know Which One) Were No Surprise
In cased you missed it, fecal transplant — use of poop from a healthy donor, which is then infused into the colon either from above (nasogastric tube) or below (colonoscope) — is unquestionably the most effective treatment for people who have multiple recurrences of C. difficile colitis (C diff).
We know this because of a randomized study just published in the New England Journal of Medicine. Here’s the punch line:
The study was stopped after an interim analysis. Of 16 patients in the infusion group, 13 (81%) had resolution of C. difficile–associated diarrhea after the first infusion. The 3 remaining patients received a second infusion with feces from a different donor, with resolution in 2 patients. Resolution of C. difficile infection occurred in 4 of 13 patients (31%) receiving vancomycin alone and in 3 of 13 patients (23%) receiving vancomycin with bowel lavage (P<0.001 for both comparisons with the infusion group).
A slam dunk win for fecal transplant, so much so that there was no point even completing the study. In the New York Times right now, coverage of the paper is actually their most e-mailed story, and even the non-clinicians in our office are buzzing about it.
(Inevitably with giggles and jokes. This stuff is hard for people to talk about, but they somehow can’t resist.)
But many of us in the ID world knew that fecal transplant was going to work great for C diff even before this study. How did we know?
- The current treatment for recurrent C diff is terrible. Metronidazole, vancomycin, and fidaxomicin all share a basic problem. They are antibiotics. And antibiotics cause C diff to begin with. Fail.
- Probiotics don’t do much. As much as I’d wish to say otherwise, the efficacy data for probiotics in preventing C diff relapse are marginal at best. Remember, lots of what makes up “normal” flora can’t even be cultured — so how do you put those bugs in a capsule? Looks like we need to use the real thing to get those normal bacteria back.

- The published uncontrolled studies on use of fecal transplant were extraordinarily favorable. In this systematic review, the cure rate was 92%. And remember, the people referred for these procedures were horribly weakened by recurrent C diff, and many had severe underlying medical conditions.
- Our limited anecdotal experience confirmed that this actually works. One of the local gastroenterologists has been doing this for a couple of years. Our first referral was an 84-year-old man with two forms of cancer, diabetes, and 5 episodes of recurrent C diff, bad enough that he’d lost more than 15% of his baseline weight. One fecal transplant procedure — C diff gone.
- For patients and doctors to keep doing something this disgusting, it must be effective. There’s a reason we snicker and jest when discussing fecal transplants — it’s gross and makes us uncomfortable. Just look at all the various euphemisms out there for this procedure — intestinal microbiota transplantation, fecal biotherapy, bacteriotherapy, human probiotic infusion. (That last one is my personal favorite — you can even call it “HPI.”) Anything to get rid of that nasty image of having other people’s poop inside of us, yuck. Sorry for mentioning that.
So consider the publication of this landmark article as a way of getting the word out to the rest of the world that this unsavory — but undoubtedly very effective — treatment is here to stay.
Let’s just hope they can find another important job for C diff Cliff.
January 16th, 2013
More Evidence That Early HIV Treatment — REALLY Early — Is Beneficial
 Management of recently acquired HIV infection — especially acute HIV, pre-seroconversion — has long been controversial, with the risks and benefits of treatment versus observation debated now for nearly two decades.
Management of recently acquired HIV infection — especially acute HIV, pre-seroconversion — has long been controversial, with the risks and benefits of treatment versus observation debated now for nearly two decades.
(Yes, it’s been that long since the publication of this controlled trial of zidovudine monotherapy. Amazing.)
On the risk side of the equation is the toxicity of lifelong treatment, especially since some patients may have normal or near-normal CD4 cell counts after recovery from acute HIV, and may remain asymptomatic for many years. Benefits include preservation of immunologic function, reduction in transmission risk to others, and even potentially a reduction in the HIV reservoir — the last making patients who are treated for recently acquired HIV of particular interest for HIV cure strategies.
Now, two studies have been published in the New England Journal of Medicine that strongly suggest that we manage early HIV infection the same way we do long-established disease — that is, with antiretroviral therapy (surprise!), and the sooner it’s started, the better.
- Le and colleagues conducted a prospective observational study involving 468 patients (95% men) in San Diego with acute or early HIV infection — 213 who initiated ART within 4 months after HIV acquisition and 384 who did not receive it until later. Sixty-four percent of patients treated early achieved the primary endpoint (CD4 recovery to ≥900 cells/mm3 during 48 months of observation) versus 34% of those treated later (P<0.0001). For those treated later, the median time to CD4 count < 500 — a threshold at which the clinical evidence of treatment benefit becomes much stronger — was only 12 months, suggesting that even if treatment is not started early, it is likely to be needed relatively soon.
- In the open-label SPARTAC Trial, conducted at sites in Europe, South America, Australia, and Africa, 371 patients (60% men) with primary HIV infection (variously defined) were randomized to receive 12 weeks of ART, 48 weeks of ART, or no ART (standard of care). The primary endpoint was either a CD4 count 3 or initiation of long-term ART. During a median follow-up of 4.2 years, only the 48-week treatment group demonstrated a delay to CD4 count 3, with 29% falling to this level versus 40% of the 12-week group and 39% of the no-ART group. The proportion who eventually went on long-term treatment initiation was similar among groups. In addition, 36 weeks after the end of short-course therapy, the mean change in HIV RNA level from baseline was significantly greater in the 48-week group than in the no-ART group (difference, –0.44 log10copies/mL). Among participants in the 48-week group, treatment started closer to the time of HIV acquisition appeared to confer greater benefit. (Quick aside — HIV-specific CD4 or CD8 immune responses did not correlate with the outcome, raising questions about whether these laboratory-based findings have any clinical relevance, or are mere associations.)
There are of course limitations to these studies, most notably the non-randomized design and skewed demographics in the first, and the fact that treatment interruption makes both of the treatment strategies in the second study of limited relevance today. Furthermore, the various definitions of early HIV infection — a problem which has plagued this field from the outset — imply a very heterogeneous patient population: In the second study, some participants had acute symptomatic HIV infection pre-seroconversion at one extreme, and others were diagnosed solely based on a newly positive HIV antibody. And neither study had sufficiently long follow-up to provide hard clinical endpoints of the benefits of early treatment.
These limitations notwithstanding, there is a consistent message from the aggregate results, which is that treatment of early HIV infection — and the earlier the better — is associated with significant improvements in the most important surrogate markers of HIV disease, the CD4 cell count, and the HIV viral load. For these outcomes alone, early treatment is warranted — especially since we’re generally recommending treatment for everyone else with HIV already!
January 11th, 2013
How to Make the Flu Vaccine More Popular, Warts and All
In a week that saw both our hospital’s influenza-induced bed crunch make the New York Times, and my son, mother-in-law, and me succumb to this seasonal plague despite our receiving flu shots, I have been highly attuned to all things influenza.
But the focus here will be on that perennial whipping boy of preventive Infectious Diseases, the flu vaccine. Let me get it out of the way that I unequivocally believe that it’s our best way of preventing flu, and that I would never skip it. Nonetheless, it’s far from perfect, which is why in the court of public opinion, it just can’t win.
Why do most universal flu vaccine campaigns seemed doomed from the outset? Let me list the ways, some of them the fault of the vaccine, some just human nature:
- It has to be repeated yearly. Like magazine subscriptions renewals, membership dues, car inspections, and taxes, the flu vaccine comes round every year and induces the same feelings of nauseating weariness. Again? You mean I need that shot already? Didn’t I just do it? And will it even work this year? All right, go ahead. Medically savvy patients note that we’re not making them repeat any of the other shots yearly, which must imply to them a serious weakness in our vaccine.
- It doesn’t always work. Estimates of flu vaccine effectiveness vary widely, so I like to cite the 70% figure from this study rather than the more discouraging CDC’s aggregate data of 60%. And then I add some motivating language such as, “but it works better in some people than others, so let’s be on the optimistic side.” Plus I mention that all doctors and nurses get the vaccine, which though a bit of a lie, is at least true about ID docs/nurses.
- Many people think it works even less well than it does. Flu season just happens to coincide with the seasonal increase in all kinds of other respiratory tract infections, and it’s inevitable that patients with bad colds will blame that on the failure of the flu vaccine. Plus, approximately 32.56% of the population use the term “stomach flu” to describe various short-term gastrointestinal ailments, and regardless of what that term actually means, it’s 100% certain that the flu vaccine does nothing to prevent them. More failure! Even the above-mentioned Times article compounds this confusion by starting the piece on influenza, yet including several paragraphs on norovirus — just because, um, it’s sort of related, I guess. (It’s not.)
- “It gives me the flu.” I find it truly mystifying why so many people believe that the flu vaccine gives them the flu, but regardless, it’s either the top reason cited by vaccine refuseniks or a close second to, “I never get the flu, so I don’t need it.” My hunch is that people believe the flu vaccine gives them the flu because 1) colds are common and 2) they once got a bad cold (the “flu”) shortly after getting the vaccine. QED.
- It has to be matched each year with the predicted circulating influenza viruses. Sometimes the match is a good one (this year it is), sometimes less so. Unfortunately, in the absence of an all-knowing, popular Nate Silver-like projection guru — someone who updates his/her predictions on a regular basis for the public to see, and does it in plain language — the process of matching the vaccine to the circulating viruses is not for the faint of heart. (I dare you to read this all the way through.) This stuff is rough going even for most doctors, hence it’s no wonder that the public is skeptical on an annual basis. For the record, this is what’s in this year’s vaccine (in case you want to make one at home): an A/California/7/2009 (H1N1)pdm09-like virus, an A/Victoria/361/2011 (H3N2)-like virus, and a B/Wisconsin/1/2010-like virus (from the B/Yamagata lineage of viruses). Piece of cake.
- Weird stuff happens. Just like no vaccine is 100% effective, none is 100% safe either. The sheer volume of flu vaccines given every single year means that bad stuff is numerically more likely to happen with the flu vaccine than with others, at least in adults. Some of these linked events will be little more than anecdotes — do you really think your golf game was worse this week is because you just got a flu shot? — but some will be real, e.g. the 1976 swine flu vaccine and Guillain-Barré syndrome. These associations are hard to shake, and not a year passes where I’m not asked about the flu vaccine and Guillain-Barré, along with much more tenuous (really nonexistent) links such as flares of multiple sclerosis, transplant rejection, arthritis, and inflammatory bowel disease.
Given the above challenges, what can we do about it?
Seems what we need is a truly trustworthy spokesperson for the flu vaccine, someone who will take on what Magic Johnson did for HIV. He or she will calmly recommend the vaccine, reassure people about its safety, acknowledge fears and misconceptions — perhaps even mention that even if the vaccine doesn’t work, it probably makes the breakthrough case of flu milder — how many people know that?
So with the help of this nice list of the most Trustworthy Celebrities, plus some of my regional/personal biases, I ask you to help choose our Flu Vaccine Celebrity Spokesperson. I am 100% sure that after hearing of their nomination, they will quickly drop whatever project they are doing and volunteer their time for this important public health effort.
January 9th, 2013
HIV Exceptionalism is Alive and Well — and That’s Too Bad
Email exchange with a colleague who works at one of our community health clinics:
Guy: Hi Paul, your patient 17432862 [that’s a made-up medical record number] came to our walk-in clinic with a rash on her hand. OK that I gave her a week of topical steroids? I know how inhaled steroids interact with some meds — wasn’t sure about the creams.
Me: Could be, good thought. Regardless, a short course should be fine. Tell me, who is the patient? What meds is she on?
Guy: I didn’t want to put her name or meds in the email, what with her disease state, HIPAA, etc.
At which point I entered the medical record number into our electronic medical record, figured out who the patient was, and let out a big sigh.
Hey, any sensible clinician understands the importance of patient confidentiality, but at some point these mysterious messages get kind of ridiculous. (I should emphasize that we have a secure internal email system.)
Furthermore, there are several reasons why the above brief email communication is a prime example of how HIV is still treated differently from the rest of the diseases — infectious or otherwise:
- It’s a patient well known to me, yet there’s no identifying information except for the medical record number. Not a first name, not even initials! Hey, we both know her, right? This will not be a disclosure of her diagnosis!
- The clinician correctly identifies a possible drug-drug interaction with HIV meds, but doesn’t actually mention any of the antiretrovirals. Too incriminating?
- When I ask for the patient’s name, he cites as the reason for not including it her “disease state” — wonder if that state votes red or blue? — with no mention of HIV.
- There’s a reference to “HIPAA, etc.”, as if HIPAA was created way back specifically for HIV. Wrong.
If we imagine that my patient had diabetes, or hypertension, or even HCV, then none of this covert communication would have occurred, I guarantee it. The implied message in all of this clandestine language, even though we both know the patient, is that there is something shameful about her being HIV positive, something that even health professionals need to communicate about in hush-hush terms.
Could this and related behaviors perpetuate the stigma associated with HIV? You bet.
Because privacy is one thing. But this is different, and kind of sad.
January 3rd, 2013
What’s a Fulyzaq? I Thought You’d Never Ask
As Physician’s First Watch noted, we sure know what the folks at the FDA were doing this holiday season — and most emphatically they weren’t visiting Aunt Selma in Boca Raton.
Nope, they were stuck in White Oak, Maryland, reviewing various new drug applications, with three of the four related to Infectious Disease. The FDA’s late 2012 actions:
- Approved use of oseltamivir (Tamiflu) in children as young as 2 weeks old.
- Approved the oral anticoagulant apixaban (Eliquis) to reduce risk of stroke for patients with atrial fibrillation not caused by valvular disease. (That’s the non-ID one — unless atrial fibrillation is caused by XMRV.)
- Approved bedaquiline (Sirturo) as part of combination therapy to treat multi-drug resistant tuberculosis. (This is is the first new TB drug approval in at least a thousand years. I’m exaggerating slightly.)
- Approved crofelemer (Fulyzaq) for diarrhea due to antiretroviral therapy.
For crofelemer, according to the FDA announcement, approval was based on the results of a phase III, double blind study comparing 125 mg of crofelemer twice daily to placebo in HIV patients with non-infectious diarrhea:
The trial was designed to measure clinical response, defined as the number of patients who had two or fewer watery bowel movements weekly. Results showed that 17.6 percent of patients taking crofelemer experienced clinical response compared with 8 percent taking placebo.
Further information on the drug — including its mechanism of action and nifty source, “the Croton lechleri plant, native to northwestern South America” — can be found on the company’s website.
I confess this approval took me by surprise. I was aware that crofelemer was in clinical trials, but hadn’t heard a peep about it at scientific meetings or other venues. Obviously some of this low profile is due to the fact that it’s not an antiretroviral agent, but compared with the attention given to tesamorelin, this is certainly a stealth approval.
Notably, current HIV medications are far less likely to cause diarrhea than the first generation of meds. The big offenders — ddI, full-dose ritonavir, nelfinavir, amprenavir, soft-gel saquinavir, even lopinavir/r — are today used either infrequently in the United States or not at all. (Several are not even available.) I assume that clinicians who have patients with ongoing diarrhea on any of these agents would switch antivirals first rather than starting a new medication for the side effect.
So who are the appropriate candidates for this drug? Even after switching off older HIV meds, some patients will continue to have diarrhea, either still due to the medications — most notably the PI’s with ritonavir — or from “HIV enteropathy”, which is damage the virus has done to the GI tract which in some people reverses very slowly, or not at all. For them, this approval gives them a welcome new option.
Plus, we get a sparkling new brand name to learn, and to make jokes about. This one’s a doozy — Fulyzaq!
So who came up with that? See above for an artistic rendition of a Fulyzaq in the wild.
January 1st, 2013
HIV Incidence: The Latest Numbers
The CDC has recently issued the latest report on HIV incidence (i.e., new infections) in the United States, and as always it’s fascinating to review the numbers.
To start, the year-by-year estimated incidence:
2007: 53,200
2008: 47,500
2009: 45,000
2010: 47,500 (38,000 men, 9,500 women)
Nope, not much change. Will data from HPTN 052 — published in the summer of 2011 — eventually have an impact? I think so, as the results strongly influenced both treatment guidelines and patients’ willingness to start therapy. A more pessimistic view is that the treatment-as-prevention message from 052 won’t have much of an effect, since data like these show that only a minority of people with HIV are actually on successful treatment. We’ll see.
This latest CDC report then highlights two important trends, one good news, one not-so-good:
The new analysis also finds two noteworthy trends among heavily affected populations: early signs of an encouraging decrease in new HIV infections among black women (21 percent decrease between 2008 and 2010), and a troubling and continuing increase in new infections among young gay and bisexual men (22 percent increase over the same time period).
In these days of fiscal cliff wrangling, I could use my taxpayer’s prerogative and show you a whole slew of figures — but these three really stand out as giving us a snapshot of who gets HIV in the United States these days:
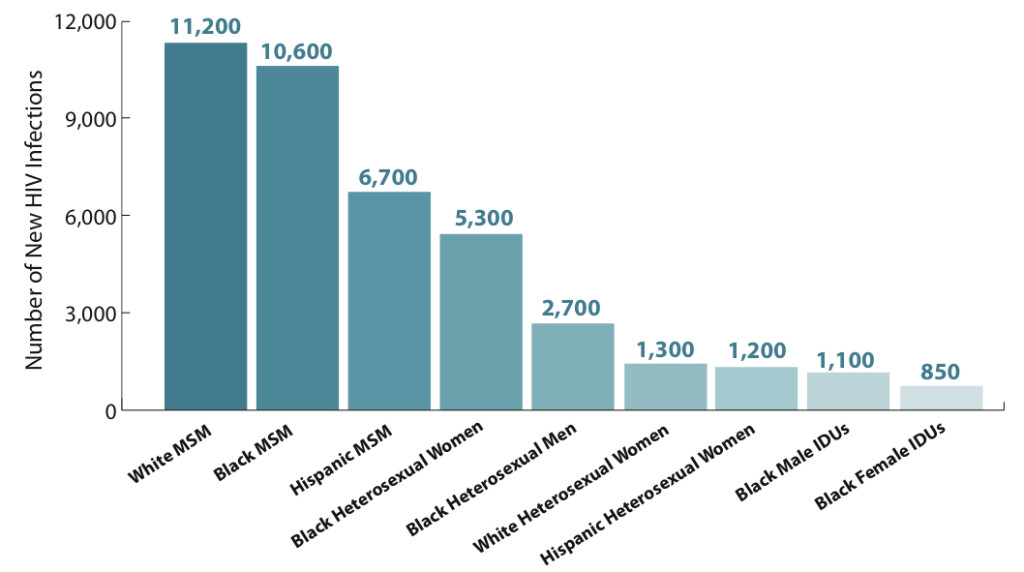
1. Incidence in 2010 among various “risk groups” (MSM, men who have sex with men; IDUs, injection drug users):
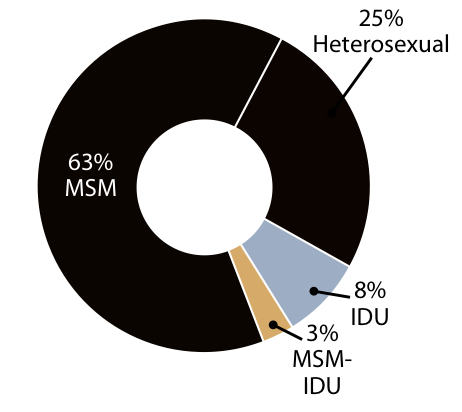
2. Proportion of new infections by transmission category:
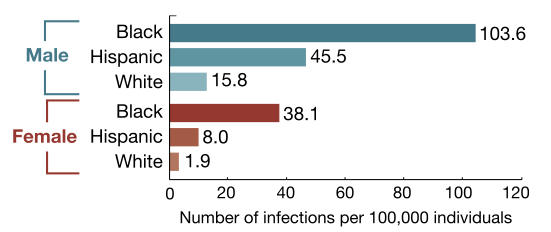
3. Rate of new infections by race, ethnicity, and sex:
The glass-half-full message is that HIV prevention efforts are clearly working, at least to some extent, as the prevalence of HIV — the number living with the virus — continues to increase, yet the incidence stays the same.
The half-empty view? The continued shift of the epidemic to younger MSM of color will make additional gains difficult, as this is a patient population that could be challenging to reach.
December 22nd, 2012
Chaos in the Diagnosis of C diff, and Dogs are Amazing Creatures
If you’re confused about the best way to diagnose C diff these days, welcome to the club. There are all kinds of tests out there, and no uniform approach between labs. Our lab actually does three tests — and will do a fourth (the classic cytotoxicity assay) if you request it.
The result? Chaos, confusion, and lots of queries.
For an example, here’s an email from a colleague:
Hey Paul — my patient had diarrhea after antibiotics, so I sent a C diff. Not sure what to tell her about the result, which I’ve pasted below. (She’s better now by the way with no treatment.)
AudreySpecimen Type: STOOL
C. DIFFICILE ANTIGEN/TOXIN ASSAY – Final
ANTIGEN:POSITIVE; TOXIN:NEGATIVE
RESULTS PENDING PCR ANALYSIS.
TOXIGENIC CLOSTRIDIUM DIFFICILE QUALITATIVE BY PCR — NEGATIVE
My response:
Audrey — the lab does two rapid tests initially — antigen and toxin assay. She had a positive antigen (indicating the presence of the organism) but a negative toxin (not all C diff produces toxin). So the PCR was done as a more sensitive and specific test for toxin, and this was negative. Strongly suggests she does not have active C diff. No treatment required. Paul
Yes, this is tricky. Some labs go right to PCR as the first step, but this is more expensive — but it sure would eliminate the confusion.
Or we could just start using dogs. As demonstrated in this paper, and summarized in Journal Watch ID by Stephen Baum, a trained dog does a terrific job of diagnosing people with C diff, even without a specimen:
A 2-year-old male beagle was trained to identify the odor of toxin-producing C. diff and to sit or lie down on detection of this scent. In preliminary testing involving 50 positive and 50 negative stools, the dog’s sensitivity and specificity were 100% …[In further testing done in the hospital], the dog correctly identified 25 of the 30 case-patients (sensitivity, 83%) and 265 of the 270 controls (specificity 98%).
The most amazing thing about this study is not only the performance of the poop-sniffing dog, but the fact that he most likely enjoyed doing it. He’s a dog, after all.
Don’t believe me? Just Watch these two video clips. First, one from the BMJ paper (let’s hope that the beagle took his HIPAA training class seriously):
And second, this Cat vs. Dog short, which clearly demonstrates (around 1 minute in) why a dog is eager to help us diagnose C diff:
December 20th, 2012
Severe Telaprevir Rashes and Waiting (or Not Waiting) to Treat Hepatitis C
Yesterday, the FDA issued a drug safety alert about severe rashes — “some fatal” — in patients treated for HCV with interferon, ribavirin, and telaprevir.
The culprit, of course, is the telaprevir. The label already contained warning information about serious skin rashes with the drug, and this alert serves to heighten our awareness of the problem, with strong wording about the importance of stopping treatment when severe rash with systemic symptoms occurs (bolding is from the FDA):
When any of these serious skin reactions occur, it is necessary for healthcare professionals to immediately stop all three components of Incivek combination treatment and the patient should receive urgent medical care.
Anyone who has prescribed or taken this drug already knows its no picnic, and of course interferon and ribavirin are hardly a walk in the park either. (Can one mix the picnic and walking in the park metaphors? Sure, why not — a picnic in the park.)
Importantly, the FDA alert again raises the key question facing people with HCV and their providers right now: Given the rapid pace of HCV drug development, with safer and more effective regimens on the horizon, why should anyone be treated for HCV today rather than waiting a few years?
I’ve asked my ID and GI colleagues this question numerous times, and received a variety of answers, but these two are the most common responses:
- Patient has advanced liver disease and can’t wait. Who knows when the new regimens will actually be available anyway?
- Patient does not have advanced liver disease, but really REALLY REALLY wants treatment now.
I certainly can understand the former, and have treated patients in this category. But what about the second one? Is this a good enough reason to treat someone with a potentially toxic treatment that, in all likelihood, will be all but obsolete relatively soon?
Anecdotally, it seems that the GI doctors (at least those that manage HCV) are more prone to treat because the patient really wants it than the ID docs. It could be our experience with HIV treatment influencing us — we saw what happened with that first generation of HIV drugs (efficacy just barely justified the toxicity, and certainly not in early disease), and we rarely use any of those medidcations today. By contrast, the GI docs see and manage advanced liver disease all the time, and must be motivated to do anything possible to prevent it.
So what do you think?
December 17th, 2012
On Service — But Some Works in Progress
When several of my colleagues attend on the inpatient consult service, they turn on an “out of office” message that provides an automated e-mail reply that goes something like this:
I am currently attending on the inpatient consult service. During this busy time, I may not be able to respond to email in a timely fashion. If you need to reach me urgently, please page my by calling xxx-xxx-xxxx, or leave a non-urgent message here and I will respond shortly.
Thank you,
Rudolph
Of course what they really mean is:
Please leave me alone.
Thank you,
Rudolph
p.s. In other words, I’m too busy to respond to you — but when I do, you should feel grateful, because after all, I told you I was busy.
p.p.s. I wish my name were something less silly.
 Now I don’t do this — though I can understand why some people choose to do so, especially researchers for whom this time is their only clinical work. (Of course those who do clinical work full time are always “on service”, but that’s a different topic.)
Now I don’t do this — though I can understand why some people choose to do so, especially researchers for whom this time is their only clinical work. (Of course those who do clinical work full time are always “on service”, but that’s a different topic.)
But there’s no doubt that regardless of who you are and what you do, if you’re in an academic medical center and on service, you will now have several additional hours of clinical work and teaching added to your day. This not surprisingly takes time away time from other activities.
Like this site.
So since I’ve been on service recently, my writing here has been relatively less frequent. In compensation, I’ll take a page from one my favorite writers, and provide a list of works in progress. Helps organize my thoughts, too.
Here’s what I’ve been working on, and what I hope will be coming out soon:
- Why HIV social workers are the greatest
- The microbiome is hot
- Another “Brush with Greatness” (it’s part of a series)
- Missing the diagnosis at case conference
- Things ID fellow applicants say, and why
- The top papers in HIV — the early years
- Clinical diversity in ID, and why we love it
- An incredibly disgusting source of infection (not sure I can even mention it on this family-oriented site)
December 5th, 2012
Top HIV Stories of 2012
Somewhere in our genome, we are programmed to use the end of the year as a time to reflect on the previous 12 months — and to make lists!
If you don’t believe me, there’s barely a publication or web site out there that hasn’t already succumbed, and we’re just in early December.
And what we can learn from these lists! For example, did you know that the the #4 search for 2012 on Bing was Gangnam Style Dance? I did not — confess I didn’t even know what it was before today — but the video below has had nearly 900 million views. Key question: Can it get to a billion with our help?
Regardless, Journal Watch/AIDS Clinical Care is not immune to this annual List Mania, and hence I’m pleased to present our Editors’ picks for the Top Stories of 2012. As usual, we select those stories that have the greatest clinical impact, or are particularly ground-breaking in their public health or pre-clinical findings. This year we’re limiting it to published papers, guidelines, and approvals rather than meeting reports and press releases.
Some of the published papers have obvious direct relevance today, such as the studies on and the FDA approval of co-formulated TDF/FTC/EVG/c (“Quad”), and its subsequent listing in HIV treatment guidelines; ditto FDA approval of TDF/FTC for PrEP. By contrast, the data on dolutegravir are likely to become even more important next year, with the anticipated publication of studies already presented at major meetings, and the likely FDA approval of this drug. And the study on “waking up HIV” is an early salvo in what should be a growing body of literature on HIV cure strategies.
Without further ado, here are the Top (published) Stories of 2012:
2. Tenofovir/FTC Approved for PrEP . . .
3. Undetectable VL – not a guarantee against transmitting HIV
4. HIV and the clinical manifestations of accelerated aging
5. Even at high CD4 counts, mortality high in untreated HIV
6. The latest “vital signs” on HIV epidemic
8. No more excuses: VL monitoring lowers costs
9. HCV is an emerging epidemic among HIV-positive MSM
10. Waking up HIV
Now it’s time to dance: