An ongoing dialogue on HIV/AIDS, infectious diseases,
August 19th, 2016
Pre-Vacation Scramble
 If you search “Are vacations good for you?,” you’ll find overwhelming support for time off.
If you search “Are vacations good for you?,” you’ll find overwhelming support for time off.
It’s as if journalists and the travel industry were in cahoots, together trying to urge us to take vacations, the longer or more frequent the better.
Hey, I get it. Time to reconnect with family and friends, to recharge those batteries, to get a fresh perspective on both work and life, to explore new challenges … yadda yadda yadda. The list of benefits is so long that it seems cliched to write them, so I’m going to stop there.
But you know what? That pre-vacation scramble to make sure things are settled just right before you go — that’s no fun at all.
And if you’re a typical doctor/nurse/PA, you probably are plenty detail oriented. ID doctors in particular will know that “detail oriented” barely begins to describe their obsessive attention to the minutiae of their everyday practice and life — and that includes establishing whether the culture was positive for M. abscessus or M. abscessus subspecies bolletii, making sure the out-of-office message conveys just the right information about when/where/how long that time away will be, and whether they’ve left clear directions to the person they’ve hired to feed the cat.
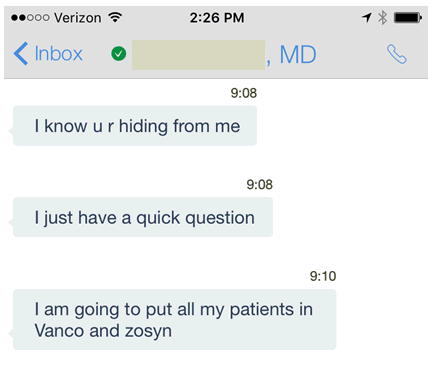
After all, how can you go on vacation if you’ve still not figured out how to get Mrs. Smith’s vancomycin level between 15 and 20? Or informed Mr. Jones that the last viral load that came back at 23 copies/mL (instead of “not detected,” as usual) is no cause for alarm?
The Big Secret, of course, it that nothing is ever completely settled, whether we go on vacation or not. So we may as well take some time off, which is what I’m about to do (hence this post).
But it also gives me a chance to share this funny (at least I think it’s funny) text, sent to me by an ID colleague who runs the Antibiotic Stewardship Program at another hospital. For non-ID readers out there, these programs (generally run by ID doctors) aim to guide clinicians to the most rational use of antibiotics, in particular to avoid inappropriate use of broad-spectrum therapy, reducing the risk of bacterial resistance (a very bad global problem).
He was scurrying around trying to finish up some work before leaving on vacation, and received this series of messages from a hospitalist who was trying to consult him one last time before he left:
Safe to say he responded to her at 9:11!
August 15th, 2016
Just Wondering: Quick ID Questions to Ponder On a Hot Summer Day
 On a lazy, brutally hot summer day, here are some more “quick questions” to think about as you hope for a cool breeze to bring relief from the stultifying (love that word) heat:
On a lazy, brutally hot summer day, here are some more “quick questions” to think about as you hope for a cool breeze to bring relief from the stultifying (love that word) heat:
- How soon will we be able to look back at contact precautions for MRSA and VRE and laugh at our folly?
- Are we again recommending antibiotics after incision and drainage of uncomplicated skin abscesses? Hard to keep track of this one. Maybe because there’s no right answer for everyone.
- What’s the best shorthand/abbreviation for “Fourth-Generation combination HIV-1 antigen/HIV-1/2 antibody immunoassay?” Boy I’m tired of writing that. How about, “HIV screening blood test” (with all the rest of the stuff implied)?
- What proportion of those with a diagnosis of both Lyme and bartonella actually have neither?
- If dalbavancin and oritavancin weren’t so expensive — say, $500/course instead of $3000-5000 — how much would we use? (I think a lot.)
- If Sporothrix schenckii had a different name, would such a high percentage of clinicians remember its association with rose thorns? “Sporotrichosis” just sounds like a disease you get from something sharp and prickly. Oh, and sphagnum moss (not sharp and prickly) and cats (could be) are also sources, for you trivia buffs.
- What percentage of our ID consult notes are actually read by surgical consultants? Regardless — what do they think of them? Especially the really long ones.
- In community acquired pneumonia severe enough to require admission, how often are antibiotics stopped after a specific viral infection is diagnosed using molecular studies? Seems to work in kids and adults for “respiratory infections”, but for admitted patients with pneumonia? Unclear, but I’d still value the information.
- Will anyone ever figure out what is causing that outbreak of Elizabethkingia anophelis in the Midwest?
- Why isn’t there more drug development for non-tuberculous mycobacteria? Gosh darn it, there’s a clinical need here. Hey there, you medicinal chemists, lab-based ID scientists, and PhDs, get on it!
- The HIV primary care guidelines recommend “anal pap tests” as a screen for anal cancer — but how often should they be done? Can anyone in good conscious suggest a test be done annually (for example) that hasn’t yet been proven to prevent cancer or improve clinical outcomes?
- Will there ever be a flu vaccine you don’t need to repeat every year? I can dream, can’t I? Or lower hanging fruit — a better mumps vaccine?
- Who will figure out how to make an ID-approved, well-done hamburger that doesn’t taste like charred sawdust?
- In the sofosbuvir combination treatments for HCV, which of the two drugs should be said first? I’ve been saying “LDV-SOF” (spelling out the “LDV”) and “VEL-SOF” (saying “VEL”), but have noticed all kinds of variations. You could do the brand names, but please. The direct-to-patient ads for LDV-SOF are almost as common as those for razors and pickup trucks.
 With all the controversy about the Rio Olympics, Zika, and contaminated water, is there any ID-related sports story more bizarre than the leptospirosis-poisoned tennis player? Hey — it gives me first-time chance to link the British Sun, that fine example of responsible journalism. Am sure they would welcome the web traffic from here, though perhaps be a bit surprised to get it.
With all the controversy about the Rio Olympics, Zika, and contaminated water, is there any ID-related sports story more bizarre than the leptospirosis-poisoned tennis player? Hey — it gives me first-time chance to link the British Sun, that fine example of responsible journalism. Am sure they would welcome the web traffic from here, though perhaps be a bit surprised to get it.- How many of those “antigen-positive, toxin negative, PCR positive” patients treated for C diff really don’t have C diff at all? If you don’t know what I’m talking about, read this.
- Will I ever remember — without struggling to come up with the specific name — that Haemophilus aphrophilus is now Aggregatibacter aphrophilus and Aggregatibacter segnis? Some facts just might be for nimble young minds only, but am making this one my personal obsession.
- Will TAF/FTC work for PrEP? Good question for a clinical trial!
- Is there anything more predictable than 1) a scientific paper finding bacteria on some household item, then: 2) the media getting all grossed out by the research, minimal clinical implications notwithstanding? Latest example — your coffee maker drip tray! Ewww!
- How long before there’s a reported antibiotic shortage do the manufacturers know it’s coming? I’m thinking about you, cefepime makers! It’s not as if demand for this drug was low.
- How many “First Zika Transmission in [insert Southern US City here] Reported” will we see this year? And how many incredibly difficult to carry out pregnancy recommendations?
- When will IGRAs completely replace tuberculin skin testing? Not saying that they are more accurate, just that they’re so much easier. Might be a downside in the healthcare screening setting — too many false-positives over time.
That’s enough for now. Need to stay cool … just like the 400-year-old shark, pictured above.
And it’s not easy being funny in 140 characters. But some people succeed brilliantly!
August 6th, 2016
Fishy, Fishy, Fishy, Fish!
I received this exciting offer recently:
Re: Fish Disease — Manuscript Invitation
Dear Dr. Paul E Sax,
Greetings for the good day!
We gladly invite you and your colleagues to contribute the articles on the topic Fish Disease in Johnson Journal of Aquaculture and Research of Johnson Publishers.
During our past two volumes, we had an excellent and fruitful cooperation, especially with our Editorial Board members.
We hope this volume will be interesting, with the presence of articles from Eminent personalities like you.
We are looking forward to receive and review your papers. For any information needed, please feel free to contact via e-mail: aquaculture@johnsonpublishers.international
Thank you for your attention towards this letter.
We are happy to announce that this is the 2nd year for this Journal. It will be a great honor for us and an excellent opportunity for you to share your newest scientific work on the field of Johnson Journal of Aquaculture and Research related issues in our international scientific review.
Regards,
Jennifer Griffin
Johnson Journal of Aquaculture and Research
Johnson Publishers
9600 Great Hills
Trail # 150 w
Austin, Texas
78759(Travis County)
E-mail: aquaculture@johnsonpublishers.international
What an opportunity! Here’s my response:
Dear Ms. Griffin:
 Thank you so much for reaching out to me about submitting a paper to the Johnson Journal of Aquaculture Research. I have long been an admirer of your journal, and am proud to say I was one of the inaugural subscribers; I eagerly await each issue with great excitement.
Thank you so much for reaching out to me about submitting a paper to the Johnson Journal of Aquaculture Research. I have long been an admirer of your journal, and am proud to say I was one of the inaugural subscribers; I eagerly await each issue with great excitement.
Your invitation today was propitious, since, as luck would have it, my research team and I have just completed a major study on the topic of Fish Disease. It should be right in your journal’s wheelhouse, as they say.
(Since you are based in Austin, Texas — Travis County, as you note — you no doubt understand that wheelhouse expression.)
Anyway, I ramble. Let me get right to the point — we think our study is groundbreaking. If we don’t win a Nobel Prize in Fish Disease (there is one in Fish Disease, right?), we’ve been robbed.
Here’s the abstract:
Background: Ichthyophthirius multifiliis (commonly known as freshwater white spot disease, freshwater ich, or freshwater ick) is a common disease of freshwater fish in home aquariums. It can cause white spots, clamped fins, and reclusive behavior due to severe embarrassment, poor little things. The optimal treatment is unknown. Methods: We randomized household guppies with moderate-severe ick to standard of care treatment (whatever that is) or a chlorhexidine whole body wash. Outcomes were assessed by trained fishologists blinded to study arm, using validated ick instruments and quality of life scores (CDC HRQOL-14, SF-36, WHOQOL-BREF, and some other letters put together that sound impressive). Results: After informed consent was obtained (at least to the extent possible from a fish), 6 guppies were enrolled, 3 in each treatment group. Baseline characteristics seemed roughly comparable, but how would we know (they’re just little guppies, after all). On a 100-point ickiness scale, the chlorhexidine washed guppies scored 22.4 International Ick Units (IIUs), vs 57.8 IIUs for the standard-of-care group (p < 0.0000001 — wow, that’s significant, isn’t it). Quality of life measures also favored the chlorhexidine-treated group (“I’m just happier,” one guppy said). Conclusion: For common aquarium guppies suffering the embarrassment of freshwater ick, chlorhexidine whole body wash is superior to usual treatment. Prevents MRSA, too.
I hope you will consider our study carefully. Eminent personalities like us ponder long and hard about the best venue for such important research. Your email was perfectly timed.
Sincerely yours,
Dr. Paul E. Sax
Henry Limpet Professor of Ichthyologic Ick
University of Travis County
(Part of an occasional series, I guess. H/T to Monty Python for the title.)
July 31st, 2016
Summertime Pre-Olympics ID Link-o-Rama
 If you’re wondering what to do between the end of the presidential conventions, the baseball trade deadline tomorrow, and the start of the Summer Olympics, here are a few ID/HIV related items to contemplate:
If you’re wondering what to do between the end of the presidential conventions, the baseball trade deadline tomorrow, and the start of the Summer Olympics, here are a few ID/HIV related items to contemplate:
- Non-travel related Zika arrives in Florida. Start getting used to seeing more of that obscure word “autochthonous”. The uncertainty was not whether Zika cases would occur here — that was inevitable — but when they would happen. Most experts still don’t think we’ll have a Brazil-sized outbreak; nonetheless, other southern states will have cases, and until we have better and more widely available tests, there will be tremendous anxiety in all affected regions. Meanwhile, we ID doctors continue to wonder why it’s so hard to get consensus on emergency funding for ID outbreaks.
- Your nose may harbor antibiotic-producing bacteria. Tons of media attention for this story! Maybe because that old favorite of ID rounds, Staph lugdunensis, is kindly providing the anti-MRSA agent, something the authors call “lugdunin”. (Not to be confused, of course, with laudanum.) This is yet another example of “colonization resistance”, a term of increasing relevance all the time.
- Training ID fellows to do penicillin allergy skin testing in hospitalized patients improved antibiotic use. It’s always such a relief to remove penicillin allergy from a patient’s problem list, especially since it’s a label usually based more on family lore than reality. (“My mother always told me I was allergic”, say even adults of advanced age.) This novel ID (not Allergy) -driven program yielded impressive implementation and outcomes data — more than half the patients who underwent skin testing then had optimization of their antibiotic regimens (narrower spectrum, more cost-effective).
- Cefazolin is as good (if not better) than nafcillin for MSSA bacteremia. Yet another study supporting the benefits of cefazolin over nafcillin (or oxacillin). The data from this and prior papers make it abundantly clear that this easier, safer treatment (cefazolin) should be chosen in most cases once patients are stable. We really do need a randomized clinical trial comparing cefazolin vs nafcillin/oxacillin for up-front therapy — who’s going to pay for it?
- FDA labels on fluoroquinolones updated to include “disabling and potentially permanent side effects of the tendons, muscles, joints, nerves, and central nervous system.” It is truly remarkable how far these drugs have fallen from their perch just a few years ago as the “Cadillac of Antibiotics” (am quoting a patient of mine commenting on ciprofloxacin — I guess even the car reference is a bit stale). It is critical to have more data on the frequency and risk factors for these rare but serious quinolone side effects, as this class of drugs is still quite useful.
- Treatment of acute HIV lowers the HIV “reservoir setpoint”, at least as measured by cell associated HIV DNA. These are the strongest data to date on this potential benefit of very early HIV treatment. I write “potential” benefit since such early treated patients are those most likely to be amenable to HIV cure strategies.
- A “microbiome” treatment for C diff failed to prevent recurrences better than placebo. Called SER-109, it is a “rationally designed ecology of bacterial spores enriched from stool donations obtained from healthy, screened donors.” It just could be that the natural stuff (ok, poop) has some unquantifiable healthy factors that just can’t be captured artificially. You know, kind of like real food vs extracted micronutrients given separately as pills, shots, whatever. Real food always wins.
- Elimination of routine contact precautions for MRSA and VRE did not increase the rate of these infections. It’s a retrospective study, and the hospitals also increased chlorhexidine bathing, but still –hospital-based clinicians are eagerly awaiting the moment when the futility of contact precautions is proven, and subsequently abandoned. Here’s hoping.
- This “patient simulation” expertly guides outpatient providers on how to avoid prescribing unnecessary antibiotics. Note that it tries to do so without making your patients mad at you, a tough challenge. One of our superb social workers is a huge fan of motivational interviewing — she most definitely would approve.
- Guidelines for treatment of coccidioidomycosis updated. If you live outside of a cocci-endemic area — which emphatically includes all of us who work in New England — good treatment guidelines and the email address of the lead author, John Galgiani, are essential items. Always makes sense to get help from those who see a lot of this sometimes serious fungal infection.
- Antibiotics for pneumonia can be stopped after 5 days, provided patients are stable and fever-free for 48 hours. Two reasons why short course therapy works: 1) The immune system is probably doing most of the work once patients start to improve; 2) Many cases of CAP aren’t caused by bacteria anyway!
- A bill added to the Massachusetts state budget would mandate insurance coverage for long-term antibiotic treatment of Lyme disease. As I’ve written before, the optimal therapy for patients with residual symptoms after Lyme treatment is anything but clear. Seems legislating coverage in an area of such clinical controversy is not the right move, and Gov. Charlie Baker fortunately agrees.
Hey, World Hepatitis Day was this past week (specifically, July 28). Do these “Diseases Awareness Days” (I made that term up) actually do anything? I can understand the motivation, but the cynic in me says that they are just preaching to the already aware. Maybe somebody has done a study of their usefulness?
Oh, and the day after was National Lasagna Day.
(Photo source: Library of Congress)
July 24th, 2016
Really Rapid Review — AIDS 2016, Durban
 The International AIDS Conference returned this year to Durban, South Africa, where it was famously first held in 2000. At that time the HIV epidemic was exploding in South Africa; funding for HIV treatment was essentially non-existent, and there was ongoing HIV denialism quite openly from some very influential figures in the South African government (including the President). Globally, fewer than 1 million people were receiving antiretroviral therapy, hardly any of them in Africa.
The International AIDS Conference returned this year to Durban, South Africa, where it was famously first held in 2000. At that time the HIV epidemic was exploding in South Africa; funding for HIV treatment was essentially non-existent, and there was ongoing HIV denialism quite openly from some very influential figures in the South African government (including the President). Globally, fewer than 1 million people were receiving antiretroviral therapy, hardly any of them in Africa.
Encouragingly, according to this UNAIDS report, the number being treated today is 17 million — with, incidentally, the largest number in South Africa. Yes, this 17 million is only half the number who need treatment, but this is still extraordinary progress. HIV-related deaths started steadily declining in 2005, a trend one can hope will continue.
OK, on to a Really Rapid Review™ of the conference. It’s organized by prevention, treatment, complications, and whatever else happened to have caught my eye; I welcome suggestions for what I’ve missed (undoubtedly something important) in the comments section.
- In the open-label extension of the IPERGAY study, the efficacy of the “on demand” PrEP was 97%. There was only 1 seroconversion out of around 300 participants, this in a patient with no detectable blood levels of tenofovir. The average number of pills taken/month was 18, so it doesn’t quite answer the question of whether this strategy works for people whose “on demand” is quite a bit less frequent than in the study participants (say, once monthly or even less often).
- In 1013 serodiscordant couples in Kenya and Uganda, 6 months of PrEP to seronegatives and ART to the those with HIV reduced HIV incidence 95% compared with historical controls. All 4 incident HIV infections occurred in people not taking PrEP, or with a source not on ART (outside the couple). Interesting question — should we really be recommending PrEP indefinitely to HIV negative people in monogomous serodiscordant relationships if their partner has virologic suppression? Our guidelines say so, but I’m dubious.
- Nearly 80,000 people have been prescribed PrEP in the USA, with over a 700% increase since 2012. The really sharp upward inflection started after presentation of the PROUD and IPERGAY studies in 2014, and not surprisingly occurred mostly in men. Fascinating geographic distribution: New York state with the largest number of scripts, then California; Massachusetts with the highest rate of prescribing based on population. Southeast USA — where HIV incidence is highest — unfortunately lags way behind, clearly a practice gap worth improving.
- A study of PrEP in at-risk 15-17 year old male adolescents showed just how hard this population will be to reach. The study pre-screened nearly 3000 peole to find 260 eligible. 152 refused participation. 108 were screened. Finally, 79 enrolled — and then 32/79 (40%) stopped the study before 48 weeks! Adherence also sharply declined, and HIV incidence was 6.4/100 person/years (that’s very high). Clearly some other strategy needed.
- The risk for drug resistance with PrEP in 5 clinical trials was only 0.05%. Even when inadvertently prescribed during acute HIV, the risk is “only” 37% — lower than I would have expected. It’s mostly M184V, of course — a mutation that would be easy to salvage.
- Could the vaginal microbiome partially explain the lower efficacy of PrEP in women? (Link is to several presentations.) Seems that Gardnerella and Prevotella spp may inactivate tenofovir more than lactobacilli. If this isn’t an ID nerd’s factoid, I don’t know what is.
- A vaccine strategy demonstrated impressive “correlates” of HIV protection. Results will allow a large efficacy study (HVTN 702) to go forward in South Africa. For the record, the strategy is called “clade C ALVAC-® (vCP2438) and Bivalent Subtype C gp120/MF59®”. These vaccine researchers sure do have their own special language.
- The PARTNERS study of “condomless sex” (published last week in JAMA) will follow MSM participants through 2018. The rationale is to provide a more precise estimate of the risk of acquiring HIV in this highest risk group, a very important remaining question.
- In a randomized study of immediate (same day) vs standard of care timing of ART in Haiti, early therapy improved survival. Important definitions: “immediate” = same day as HIV diagnosis; standard-of-care = only 21 days later, with counseling visits before then. This is a remarkable result, especially since 1) patients with active OIs were excluded; 2) the number of clinic visits was the same; and 3) the effect was so great a DSMB stopped the study early. Seems that starting ART right away improves engagement in care, and all of those “are they ready to start ART?” questions have been answered: YES THEY ARE.
- In the ARIA study, ABC/3TC/DTG was superior to TDF/FTC + ATV/r in treatment naive women. There were both fewer discontinuations for adverse events and fewer virologic failures in the ABC/3TC/DTG arm. The integrase-first strategy wins again, with a very similar outcome to the WAVES study, which used ECF-TAF and was blinded.
- In LATTE-2, an every 4 week schedule of cabotegravir and rilpivirine injections had fewer virologic failures than every 8 weeks at 48 weeks. One of the participants in the q8 week arm developed resistance to both rilpivirine and cabotegravir. Note that while both strategies were comparable to oral therapy, the q4 week approach will be used in the phase 3 studies based on these results. And in a funny mix-up showing how small the HIV research world is, the presenting author David Margolis was introduced as the other David Margolis. Bet the two John Bartletts have had a similar experience.
- In newly diagnosed patients with advanced HIV disease (CD4 < 100), adding raltegravir to standard ART did not improve clinical outcomes. Once again, 4 drug ART was not better than 3, though as expected HIV RNA declined faster and CD4 increased more in those receiving RAL. Called the REALITY study, this was an extraordinarily ambitious (sorry for the cliche, but it’s true) trial, conducted in multiple African countries and with 3 randomizations with a factorial design: intensive ART (this part), enhanced OI prophylaxis (see next bullet), and nutritional intervention (not presented here). This is the largest study done in advanced HIV disease, a group for whom we still have lots of questions since early mortality is so high.
- In this same vulnerable population with advanc
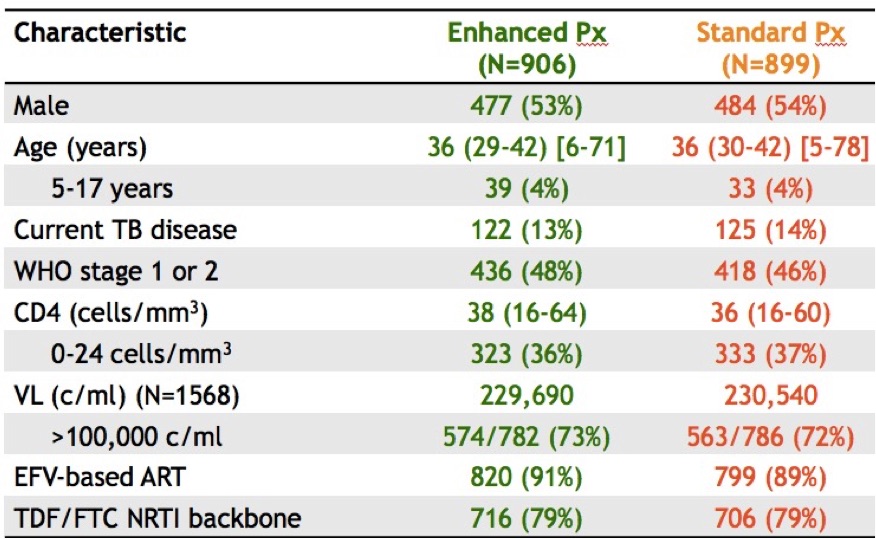 ed disease, enhanced OI prophylaxis improved survival. In addition to ART, the control arm received TMP/SMX (+/- INH depending on that local policy); the enhanced group received ART plus TMP/SMX, INH, fluconazole for 12 weeks, azithromycin for 5 days, and a dose of albendazole (why not). And take a look at those entry criteria! Mortality at 24 weeks: 8.9% vs 12.2% (p< 0.04), benefits sustained at week 48. These results could change treatment guidelines for this region; the number needed to treat/live saved was only 30. For the record, my friend/colleague Trip Gulick had a similar idea for a study of “pan-prophylaxis” in the USA — in 1991 (5 years before effective ART).
ed disease, enhanced OI prophylaxis improved survival. In addition to ART, the control arm received TMP/SMX (+/- INH depending on that local policy); the enhanced group received ART plus TMP/SMX, INH, fluconazole for 12 weeks, azithromycin for 5 days, and a dose of albendazole (why not). And take a look at those entry criteria! Mortality at 24 weeks: 8.9% vs 12.2% (p< 0.04), benefits sustained at week 48. These results could change treatment guidelines for this region; the number needed to treat/live saved was only 30. For the record, my friend/colleague Trip Gulick had a similar idea for a study of “pan-prophylaxis” in the USA — in 1991 (5 years before effective ART). - Updated 48-week results of the dolutegravir plus lamivudine (PADDLE) study had 1/20 with virologic failure. This is a single-arm pilot trial for patients with screening HIV RNA < 100k. Notably, the one with virologic failure had entry HIV RNA > 100K and was suppressed initially, but developed low-level viremia from week 36-48. No resistance was detected, and he re-suppressed on DTG-3TC and now is on triple therapy. (A second patient died from suicide before week 48 for an overall efficacy of 90%.) We need larger studies of this strategy in a broader patient population and with longer follow-up before it is widely adapted.
- A novel formulation of once-daily raltegravir was non-inferior to standard twice daily dosing. In this randomized, double-blind trial, the once daily arm was dosed as two 600 mg tablets daily, and all subjects received TDF/FTC. While overall outcomes were virtually identical (and excellent) in both arms, the once-daily arm had 5 cases (0.9%) of emergent resistance vs zero in twice daily arm. Interestingly, both arms had a lower rate of resistance than in prior randomized studies of raltegravir in naive patients.
- In START, the patients who benefited the most from early ART had HIV RNA > 50,000, or CD4:CD8 ratio < 0.5, or age > 50, or Framingham risk scores > 10%. These individual characteristics brought the number needed to treat for benefit of early ART (CD4 > 500) down from 128 to 40-50. Important fact for stat geeks — this was a univariate analysis.
- In the PROMISE Study, pregnant women with high nadir CD4s who continued ART post delivery had better clinical outcomes than those who stopped therapy. This was an important clinical question when this study was designed in the late 2000s, but the comparison had to be stopped early when the START study results became available last year. While the primary endpoint (AIDS, severe non-AIDS events, death) showed no difference between arms, those who continued had fewer other manifestations of HIV disease — TB, bacterial infections, zoster, mucosal candidiasis.
- Efavirenz again associated with suicidality in patients starting ART. This complex analysis (also from the START study) had to account for the fact that investigators avoided EFV-based regimens in participants with psychiatric disease (“channeling”), so in fact those receiving EFV had a lower rate of suicidality than those who did not. However, the rate was higher comparing EFV treated subjects to those in the deferred arm; this was not observed in other regimens. Given the results of the published ACTG study with similar findings, I would certainly avoid initial EFV-based therapy in those with a history of depression (and probably pretty much everyone if you have other options).
- In the ASTRAL-5 study, 12 weeks of velpatasvir/sofosbuvir for HIV/HCV co-infection cured 95% of study participants. This is a pan-genotypic regimen, a terrific new option for genotypes 2 and 3 in particular. Note that it cannot be given with efavirenz, and that if your patient is on TDF, a switch to TAF makes sense just as it does for ledipasvir.
- In the TURQUOISE-I, Part 2 Study, “PROD” (+/- RBV) cured 97% or more of those with HIV/HCV coinfection. (PROD = parataprevir, ritonavir, ombitasvir, dasabuvir.) Despite the relatively high pill burden and need for RBV in genotype 1a, this is a very effective regimen. Cumbersome study name, though. Couldn’t it have been “Turquoise 2”? Reminds me of this funny post on movie titles, probably could to the same thing for study names.
- Adjunctive therapy with vorinostat, maraviroc, and hydoxychloroquine did not decrease time to virologic rebound or reduce the size of the latent reservoir. This small randomized trial was conducted in patients treated during acute HIV infection — hence those most likely to benefit from cure interventions. A well done study, but probably one of many “negative” studies done in this area we’ll see over the next few years.
A few non-scientific words about being back in Durban after 16 years:
- Underrated beachfront. There’s plenty of activity during the day, with the surfers out between 6-7AM and the walkers, joggers, bikers, skateboarders, rollerbladers, and general observers appearing just a bit later to experience the beautiful sunrise. All day long a walk on the beachfront promenade was an ideal way to clear the brain of “conference head.”
- Great, affordable food. Not surprisingly, there is a pervasive Indian influence, as Durban has one of the largest Indian populations in the world outside of India. (If you get a bunny chow, be reassured it has nothing to do with rabbits.) And don’t skip the Pinotage and Chenin Blanc (I didn’t). Unfortunately, unlike my experience 2 years ago in Melbourne, the coffee is terrible.
- Pride. Every person I met from Durban was both extremely kind and extraordinarily proud of their city; all knew the history of the city well, and were eager to talk about it. I sensed a bit of both envy and disapproval of both Cape Town and Johannesburg.
- Uber rules. Cheap, reliable, and every bit (if not more) the “disruptive innovation” it is in the USA.
- Safety. It did seem as if the local advice about security was even stronger than the first time I visited. It was obvious stuff: don’t walk alone to the conference center, don’t carry your computer, don’t take out your cell phone on the street, and (repeatedly) never walk alone at night. Some of this, no doubt, is that the first time we were here it was pre-9/11. It’s a different world.
An ancillary benefit about going to this conference — the noise from a certain political convention was only a faint peep, or a footnote on a distant TV that happened to be turned to CNN!
July 3rd, 2016
Velpatasvir/Sofosbuvir Makes HCV Treatment Simpler, Especially For Genotypes 2 and 3
One of the ways ID and hepatology hepatitis C experts like to show off is by discoursing on the nuances of cleverly named clinical trials, and how these impact treatment guidelines.
It usually goes something like this:
“In the EP-CILEON [I made that up] study of [insert HCV regimen here], treatment-experienced patients with genotype [insert non-genotype 1 patients here, usually genotype 3], compensated cirrhosis, and baseline viral loads greater than [some large number], the SVR [why can’t they say “cure”?] to the 12-week regimen was only [insert some number here that we could have only dreamed about in the interferon era, but well shy of the 95% mark we expect today — let’s say “82%”]. That’s why these patients need to be treated for [a longer duration than 12 weeks, a number also divisible by 4] weeks, with the addition of weight-based ribavirin [as opposed to fixed-dose ribavirin? when would we do that?].”
Fortunately, these obscure study results are then quickly incorporated into the excellent HCV guidelines, so we mortals can just look them up.
With the FDA approval last week of velpatasvir/sofosbuvir (VEL/SOF, “Epclusa”), however, bragging rights to these arcane details might now be irrelevant, kind of like knowing how to text with a flip-phone.
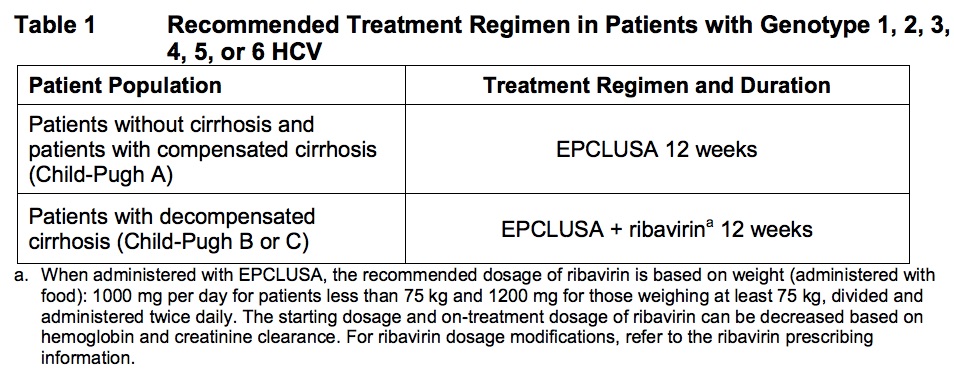
The new option makes things pretty simple, really. For patients with HCV genotypes 1-6 (that’s all of them, if you’re keeping track), here’s what to do:
- Almost everybody: One pill of VEL/SOF a day for 12 weeks.
- Those with decompensated cirrhosis (Child-Pugh B or C): Same thing, but add weight-based ribavirin.
Or if you prefer, from the package insert:
In several clinical trials (published right here in the hallowed pages of the NEJM), VEL/SOF cured 95-99% of those treated, including patients with tricky genotype 3. Side effects were infrequent, and, as we’ve come to expect with modern HCV therapies, discontinuations due to adverse events wonderfully rare (0.2%).
Of course you’re thinking — Sounds good, but what does this new treatment cost? Because that, after all, has been the major challenge in HCV therapy the past two years: Access.
The simple answer is that the price is around $75,000 for a 12 week course.
And that is potentially good news, as demonstrated by the following (approximate) list “prices” for various treatment courses, the most relevant comparisons for the VEL/SOF option bolded:
- Ledipasvir/sofosbuvir for 12 weeks, genotype 1: $94,000 (8 weeks for HCV RNA < 6 million, no cirrhosis, treatment naive, is 2/3 that cost)
- Paritaprevir/ritonavir/ombitasvir plus dasabuvir, genotype 1: $83,000
- Grazoprevir/elbasvir for 12 weeks, genotype 1: $55,000
- Sofosbuvir plus ribavirin for 12 weeks, genotype 2: $88,000
- Sofosbuvir plus daclatasvir for 12 weeks, genotype 3: $147,000
I wrote “potentially” good news, since these numbers are before negotiated prices. The negotiated price is the deal made between the pharmaceutical companies and the payers, behind closed doors and opaque to patients and providers, but quite relevant when we want to prescribe something.
Hardly any insurance plan or pharmacy benefit manager pays these full prices — it’s sort of like the sticker price on the car in the showroom, a starting point for discussion. However, it differs from buying a car in that there’s no easy internet research to find out what the plans actually pay. We prescribers and patients get a relative idea when the prescription either sails through or gets blocked in favor of something else.
But a quick glance at the clinical trials data and the sticker prices shows that VEL/SOF could easily become the recommended treatment for genotype 2 (good riddance, ribavirin!) and genotype 3. For the latter, this is not only based on price; sofosbuvir plus daclatasvir needs to be given for 24 weeks, with ribavirin, in all genotype 3 patients with cirrhosis. So VEL/SOF really is an advance — this is a simple, safe, and effective 12-week treatment for all genotypes that will rarely need ribavirin, except when there is decompensated cirrhosis (Child-Pugh 2 or 3).
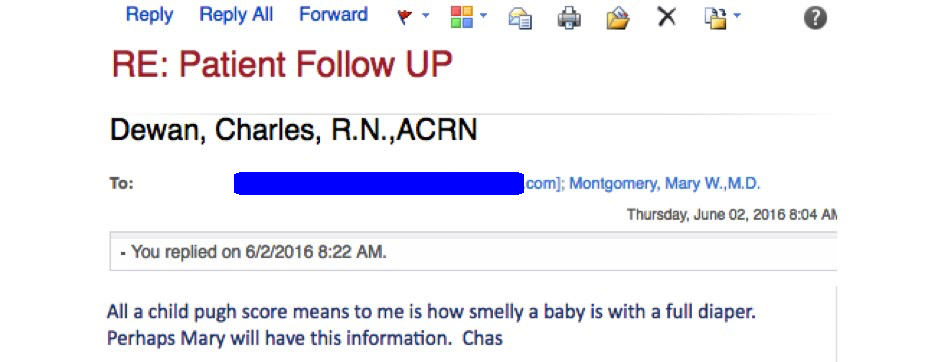
And for those who are not very hepatically inclined, and wondering what the heck Child-Pugh is, it is a commonly used scoring system to assess the prognosis of patients with cirrhosis.
Take it from Charlie, our beloved clinic nurse, who had this email exchange recently with a pharmacy over getting HCV treatment approved for one of our patients:
Such a great line, had to share.
Hey, since I started this post with a parody of sorts, here’s one on TED talks making the rounds that could not be more spot-on.
[youtube https://www.youtube.com/watch?v=_ZBKX-6Gz6A&w=560&h=315]
June 25th, 2016
ID Cartoon Caption Contest Closed — Time to Vote
The response to our First Ever ID Cartoon Caption Contest was gratifyingly brisk, with hundreds of entries.
Not going to lie about this — we were somewhat concerned the response would be silence … you know, as in <<crickets>> … but you readers proved very much up to the task, with numerous funny suggestions.
Our sophisticated computer algorithm has gone through the submissions, and noted several themes, specifically:
- Pig-Pen.
- Colistin, KPCs, and other references to resistant bacteria.
- Fecal transplants.
- How hard (and messy) it is to be an intern, resident, or fellow, compared to the clean faculty members standing next to him.
- The ironic (and therefore funny!) contrast between the coat (dirty) and his hands (presumably washed) or tie (tucked in).
- References to historic names in ID/microbiology (Lister, Petri, Semmelweis).
- The Hygiene Hypothesis.
- How being an ID (or GI, or Emergency Medicine) doctor, or Pediatrician, can be a squalid business.
- Puns/word plays on medical terminology. Many funny, all groan-worthy.
Of course, some responses were just baffling, with even our sophisticated supercomputer unable to decipher the humor — or frankly the meaning — of the submission.
The good news is that after hours of number-crunching, we’ve come up with 3 possible winners.
First, as a refresher, the picture:
Now, no doubt inspired by this wacky Presidential campaign and Brexit, you get to vote.
June 19th, 2016
ID Cartoon Caption Contest
The New Yorker magazine is justifiably famous for its fine writing, with its contributors a veritable Who’s Who of famous authors and journalists of the past century. Truman Capote, Ann Beattie, J.D. Salinger, John Cheever, John Updike, Dorothy Parker, E.B. White, Philip Roth, Alice Munro, John Hersey, Malcolm Gladwell, Roger Angell, James Thurber … you get the idea.
But for many (OK, I admit it, often for me), it’s mostly just a print vessel for the cartoons. These offer some of the most brilliant commentaries on the world you will find anywhere — witty, profound, whimsical, silly — and my web browser has this link bookmarked in case I’m feeling glum.
The magazine also has a Cartoon Caption Contest, in which readers contribute captions for a drawing that changes weekly. I’ve entered the contest dozens of times, and have won the same number of times as I’ve won Wimbledon.
Oh well. You might think this brilliant entry could have done the trick:
I share this low success rate with my sister Anne, who in my unbiased opinion should have won with this one:
Admittedly motivated by our failure, we hereby offer our own ID Cartoon Caption Contest. The drawing is Anne’s (I’ve featured her art before), and it was inspired by a certain white coat controversy.
Submit your caption either in the comments section, to my Twitter feed, or if you want to keep it secret, email me at id.caption@yahoo.com.
We’ll judge them on funniness using only the most highly objective and scientific criteria. In addition to widespread fame, the winner will receive a free lifelong subscription to this blog.
Here’s the drawing — have at it:
June 12th, 2016
Progress in Lyme Disease Badly Needed — Could a “Hackathon” Help?
Someone recently asked what keeps me, a specialist in Infectious Diseases, up at night.
With the admission that I do all my clinical work here in the USA — a person working in the tropics would undoubtedly have a different list — several challenging patient care and public health issues came to mind. Multidrug-resistant bacteria. Endovascular infections in patients with opiate addiction. Surgical infections with poor “source control”, especially in the abdomen. Non-tuberculous mycobacteria. Recurrent C diff and MRSA. Patients who, despite having access to lifesaving HIV therapy, don’t take their meds. An aggressive, transmissible flu strain arising from our close contact with birds or pigs. Aedes albopictus starts transmitting dengue, chikungunya, and Zika. Drug prior approvals that defy medical common sense. Electronic medical records that force docs to become overeducated clerks.
And, of course, Lyme Disease.
I’m far from alone about this concern, by the way. In an effort to make some progress against this challenging infection, a “healthcare hackathon” on Lyme will be held in Cambridge June 17-20, led by the Dean Center for Tick Borne Illness. And if you’re wondering what a healthcare hackathon is, one of the Dean Center’s doctors kindly shared this review — it’s essentially it’s a way of bringing together experts from multiple fields to work together and tackle a problem.
This approach makes abundant sense, as solutions to complex problems rarely come from a single discipline.
And boy, is Lyme the very definition of a complex problem, a keep-you-up-at-night topic — allow me to list why:
- Lyme has become way more common. When I became an
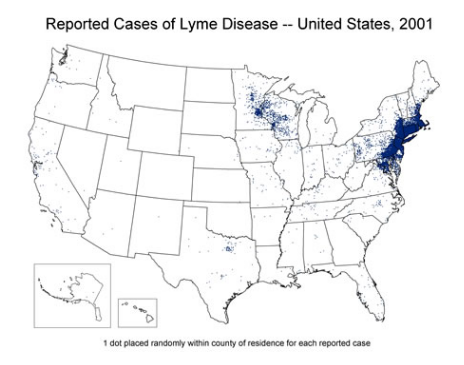 ID specialist in the early 1990s, most of the people we saw with with Lyme lived on or near the coast, or had visited Cape Cod, Nantucket, or Martha’s Vineyard; now we see Lyme acquired throughout New England, and even in urban areas. It is spreading from the Northeast and Midwest into the South, and it no longer disappears completely in the winter — all it takes is a prolonged warm spell, and a few cases pop up. Here are the numbers per CDC — there are 300,000 cases/year in the USA, a substantial increase over the last 3 decades, and no doubt an underestimate since many cases are not reported.
ID specialist in the early 1990s, most of the people we saw with with Lyme lived on or near the coast, or had visited Cape Cod, Nantucket, or Martha’s Vineyard; now we see Lyme acquired throughout New England, and even in urban areas. It is spreading from the Northeast and Midwest into the South, and it no longer disappears completely in the winter — all it takes is a prolonged warm spell, and a few cases pop up. Here are the numbers per CDC — there are 300,000 cases/year in the USA, a substantial increase over the last 3 decades, and no doubt an underestimate since many cases are not reported. - Severe Lyme Disease is a bad problem. Yes, most cases of
 erythema migrans respond promptly to therapy; there are even people with positive tests who have never been treated and feel totally fine. That’s the good news, the mild end of the spectrum. But rarely, especially (in my anecdotal opinion) for those who get sick and delay diagnosis and treatment, Lyme can be very serious — high fevers, cardiac disease, hepatitis, arthritis, encephalitis, meningitis, neuropathies, radiculitis, myelitis. And these severe cases are those most likely to have residual symptoms after treatment.
erythema migrans respond promptly to therapy; there are even people with positive tests who have never been treated and feel totally fine. That’s the good news, the mild end of the spectrum. But rarely, especially (in my anecdotal opinion) for those who get sick and delay diagnosis and treatment, Lyme can be very serious — high fevers, cardiac disease, hepatitis, arthritis, encephalitis, meningitis, neuropathies, radiculitis, myelitis. And these severe cases are those most likely to have residual symptoms after treatment. - There is no proven optimal treatment for patients with ongoing symptoms after Lyme. Some people think it’s best to try more antibiotics, targeting residual active bacteria. Others (read: most ID doctors) think that’s not a good idea, for four main reasons: 1) The scientific data on residual living spirochetes after treatment are far from definitive; 2) Several controlled clinical trials — most recently this one — show no benefit long-term antibiotic therapy; 3) Post-infectious symptoms may occur after many severe infections (bad influenza, sepsis, endocarditis, pneumonia, toxic shock syndrome), and we don’t give long term antimicrobial therapy for these conditions; 4) Antibiotics can be harmful — a colleague of mine notably had a patient who required a colectomy after developing C diff on a prolonged course of ceftriaxone prescribed by a Lyme specialist. Still — if we’re not giving antibiotics, what are we offering? To quote this excellent editorial that accompanied the above cited clinical trial — “Though prolonged antibiotic therapy is not the answer, we do not know what is truly helpful.” (Emphasis mine.) No wonder our patients are unhappy!
- Testing for Lyme is confusing, sometimes inaccurate, and slow. Think how accurate HIV testing has become — sensitivity and specificity are way north of 99%, false-positives and false negatives extremely rare, and generally quite easy to sort out with supplemental tests. HIV test results are back quickly, either right away with a point-of-care test, or at most a few days. Lyme testing is the opposite — early in disease, antibody testing lacks sensitivity; later on, the two-step procedure of screening ELISA followed by Western blot is fraught with false positives (especially IgM immunoblots) and, according to some, false negatives. Delays in receiving definitive results are common, and labs do not all have the same criteria for positivity. Molecular testing with PCR is of limited accuracy (even in acute disease), and follow-up Lyme antibody tests after treatment don’t provide proof of cure. The problems with standard Lyme testing have spawned a variety of “home brew” alternatives — here’s a terrific brief review — and note that none of them is FDA-approved, many require that desperate patients pay out of pocket, and all add to the confusion about who does and who does not have the disease. Thank you very much. (That was sarcasm.)
- Instead of cooperation, there is pervasive rancor in much of the discourse on Lyme. For whatever reason, the difficulty in prevention, diagnosis, and treatment of Lyme has created conflicts between patients, providers, and public health officials — a conflict far different from other challenging diseases. For example, the documentary Under Our Skin repeatedly attacks the medical community for ignoring the disease, and promotes numerous unproven diagnostic tests and treatments. There are several unfortunate results of this conflict, but one of the most discouraging from my perspective is the very speciality that should be front-and-center in trying to solve the problem — Infectious Disease — is repeatedly attacked; under that attack, ID doctors sometimes dig in their their heels on these controversial issues, or ignore them entirely, and the discourse stops. One can read opposing views on this from activists and IDSA here and here, respectively.
This last item (the controversy) is the reason why, for the last several weeks, a green flyer promoting the Lyme Hackathon has been posted in our clinic, and I’ve kept a copy on my desk. Notably absent from the pre-meeting materials are attacks on either side of the debate — a refreshing change from the usual harrangues.
I wish them the best of luck — we are all hoping for some progress!