An ongoing dialogue on HIV/AIDS, infectious diseases,
September 28th, 2019
What Is the Best Treatment for Advanced HIV Disease?
One of the things that keeps me on Twitter — besides cute dog videos — is the periodic realization that the platform can help patients.
Which is, after all, why most of us do this doctor thing — to help people get better.
Example:
Several months ago we had a challenging patient. I asked twitter if anyone had ever used compassionate use cefiderocol. #IDtwitter responded and help Dr. Clancy and I save this gentleman’s life. So pleased to finally share the case back where it started! https://t.co/sStAqf0xFS
— Ryan Stevens (@Stevens_AK) September 26, 2019
So let’s consider this case from the energetic and amusing Canadian ID doctor Sebastien Poulin, which he posted a couple of weeks ago.
He was kind enough to share a few more details of the case with me via email (certain details slightly changed):
Middle-aged man admitted with fatigue and weight loss. Evaluation notable for oropharyngeal candidiasis, moderate pancytopenia, intra-abdominal lymphadenopathy (mesenteric and perihepatic), homogeneous hepatomegaly without splenomegaly.
HIV test positive. CD4 13; HIV RNA 2 million. Started on TDF/FTC, dolutegravir. Also TMP/SMX for PCP prophylaxis.
Three weeks later, blood culture turns positive for M avium complex.
Two questions immediately occurred to me with this challenging case:
 Question #1: Is this this TDF/FTC plus DTG regimen the optimal choice, or should we add (or use) boosted darunavir?
Question #1: Is this this TDF/FTC plus DTG regimen the optimal choice, or should we add (or use) boosted darunavir?
Certainly TDF/FTC plus DTG is a guidelines-endorsed first-line regimen, and the clinical trials of DTG as first-line therapy demonstrate good activity across a broad range of baseline viral loads and CD4 cell counts.
But consider the three case reports of treatment failure with emergent DTG resistance in initial treatment with three-drug therapy:
- Fulcher, et al, Clin Infect Dis 2018;67(5):791-4: HIV RNA — 1,970,000. CD4 — 78. Active infection — PCP. Relevant concomitant medications — None.
- Pena MJ, et al. Open Forum Infect Dis 2019;6(1):ofy332: HIV RNA — 457,000. CD4 — 39. Active infection — Staph aureus. Relevant concomitant medications — rifampin (DTG dosed twice-daily).
- Lübke N, N Engl J Med 2019; 381(9):887-9: HIV RNA — 1,400,000. CD4 — 22. Active infection — TB. Relevant concomitant medications — rifabutin (DTG dosed once-daily). [Special note from me: got to love when OFID scoops the NEJM!]
All of these cases involved people with HIV who had advanced HIV disease, high viral loads, and active infections. All ultimately achieved viral suppression with inclusion of darunavir-based therapy.
Remember, patients like this, or the case posted by Sebastien, did not qualify for the registrational clinical trials of dolutegravir. Even stable patients with severe immunosuppression and high viral loads were uncommon. The same limitation applies to studies of bictegravir/FTC/TAF.
In contrast, consider the NAMSAL study — 66% of entrants had a viral load of ≥100,000, and 31% had a viral load of ≥500,000. Responses to both the efavirenz and DTG-based strategies was notably lower in these participants.
So how comfortable should we be using DTG or BIC-based regimens in someone with a baseline viral load of 2 million and a CD4 cell count of 13? An ongoing clinical trial might help us answer the question, but until results are available, what to do?
Question #2: Should rifabutin be part of the treatment for MAC?
In a prospective, comparative clinical trial of treatment for HIV-related disseminated MAC, the three-drug regimen of clarithromycin, ethambutol, and rifabutin improved survival compared with either clarithromycin plus ethambutol or clarithromycin plus rifabutin.
So the answer to this Question #2 seems pretty obvious. Yes!
But we might wonder whether these data are still relevant to Sebastien’s case. Only 14% of the participants in that study were receiving PI-based ART. Plus, the study enrolled from 1994-1998, when combination ART was markedly less effective and more toxic than today — and was especially difficult to tolerate for patients with advanced HIV disease and active opportunistic infections.
As a result, the current OI guidelines say the following about adding a third drug for disseminated MAC:
Some experts would recommend addition of a third or fourth drug for people with HIV with high mycobacterial loads (i.e., >2 log CFU/mL of blood), or in the absence of effective ART.
You know that whenever the “Some experts” phrase makes an appearance, there are no strong data to direct us one way or another. And who these days knows a patient’s quantitative “mycobacterial load”?
Let’s look again at our cases of dolutegravir failure with resistance — two out of three received rifamycin drugs. Rifampin strongly induces DTG metabolism, which requires doubling the dose to 50 mg twice daily (the clinicians did this in case #2). Rifabutin has much less of an effect, so no change in dose is required. Still, experience with rifabutin and dolutegravir in clinical practice must be quite limited.
So back to our questions:
- Is this this TDF/FTC plus DTG regimen enough, or should we add (or use) boosted darunavir?
- Should rifabutin be part of the treatment for MAC?
Let’s do a poll on #1. Please use the comments section to let us know why you made your choice, as well as your thoughts on the rifabutin quandary in question 2.
As for cute dog videos, there’s this:
“Difficulties strengthen the mind as labour does the body.”
Seneca the Younger, Roman Stoic philosopher (4BC-66AD.) pic.twitter.com/7tnxzWbX48— Dick King-Smith HQ (@DickKingSmith) September 10, 2019
September 15th, 2019
A Former Medical School Dean Invents a False Dichotomy in Curriculum Content, and Advises Physicians to Stay in Their Lane

Aristotle, who famously said this.
Over on the editorial pages of the Wall Street Journal, a piece appeared last week with the following provocative title and subtitle:
Take Two Aspirin and Call Me by My Pronouns
At ‘woke’ medical schools, curricula are increasingly focused on social justice rather than treating illness.
Dr. Stanley Goldfarb, former associate dean of curriculum at the University of Pennsylvania, argues that current medical schools focus so much on advocacy, social justice, and various (left-leaning) causes that students don’t have time to learn how to care for patients.
And what are some of the distractions these medical students must endure as they try to master the craft of medicine?
Cultural diversity, gun control, climate change, health disparities. Teaching about these topics “comes at the expense of rigorous training in medical science.”
He cites no actual data that this is true, of course, which means it’s his opinion — an opinion I strongly suspect he harbors based on how he feels about these topics himself.
Regardless of how we feel about them, however, most will recognize the “back in my day we studied real medicine” tone. This one turns up frequently when many of us old-timers weigh in on the state of medical education today, be it in medical school or residency. You know, “back in the days of the Giants”, bringing to mind the “OLD MAN YELLS AT CLOUD” internet meme.
But back to the topic of his piece — is it really a new phenomenon that medical schools include a societal (as well as individual) view of medicine, and that students show interest in these topics?
Absolutely not — back when I went to medical school a million years ago (ok, in the early 1980s), we were urged always to consider our patients in the context of their community, and also to think broadly about what we could do as doctors to improve not just individual, but also community health. While patient care was a the core of our efforts, my classmates ended up choosing a huge range of different passions to pursue.
A quick list: Basic science research. Clinical research. Public health and epidemiology. Health disparities, both here and globally. Domestic healthcare policies. Healthcare finance. Teaching. The medico-legal interface. Investment banking and consulting. One even started her own footwear company!
In short, one of the great things about medical training is that it’s adaptable to a wide range of health-related pursuits. Since what we include in a medical school curriculum cannot possibly cover everything — more true today than ever — why not include topics that are important from a societal level too? Don’t these influence patient outcomes?
Of course they do — sometimes powerfully so, something most University of Pennsylvania doctors readily acknowledge, citing the vast economic disparities evident right there in Philadelphia.
I respectfully disagree with Dr. Goldfarb's arguments. @PennMedicine's students are required to rotate in our West Philadelphia EDs. Traditional curriculums do not prepare them to address the barriers (violence, poor housing, addiction etc) that impede good clinical outcomes
— M. Kit Delgado, MD, MS (@kit_delgadoMD) September 13, 2019
And it appears the University of Pennsylvania agrees, as evidenced by the letter they have sent to students and faculty:
Please know that the views expressed by Dr. Goldfarb in this column reflect his personal opinions and do not reflect the values of the Perelman School of Medicine. We deeply value inclusion and diversity as fundamental to effective health care delivery, creativity, discovery, and life-long learning. We are committed to ensuring a rigorous and comprehensive medical education that includes examination of the many social and cultural issues that influence health, from violence within communities to changes in the environment around us.
Additional Penn faculty quickly weighed in on the editorial, noting that “social and health policies have always determined who gets sick and who gets care, and where, and how.” Unlike in the Wall Street Journal piece, these authors include some clear examples — the Flint Water Crisis, urban gun violence, underdiagnosis of cardiovascular disease in women and depression in African Americans — on how poverty, race, and bias influence individual and public health.
As for Dr. Goldfarb’s opening salvo:
The American College of Physicians says its mission is to promote the “quality and effectiveness of health care,” but it’s stepped out of its lane recently with sweeping statements on gun control.
September 8th, 2019
The Curious Case of M184V, Part 2 — and More!
 The inspiration for today’s post comes from two recent emails from ID/HIV colleagues — thank you.
The inspiration for today’s post comes from two recent emails from ID/HIV colleagues — thank you.
Here’s the first, from Dr. Mehri McKellar from Duke:
Hi Paul,
When are you going to do part 2 of The Curious Case of M184V, Part 1?
I am waiting patiently. 🙂
Mehri
Mehri, wait no more, because here it is! And thank you for the reminder, which is quite timely since there’s now greater clarity than ever about M184V, and what to do with that next regimen.
That first post summarized why we even care about this mutation, and what makes it so special — and so curious. Specifically:
- It’s the most common NRTI mutation observed with treatment failure. HIV docs averse to memorizing mutations should definitely put this one on their short list anyway — sorry, some you just have to know.
- Despite finding it frequently in people on treatment with viremia, we rarely see it transmitted in newly diagnosed patients. Latest data had it at < 1% in the United States.
- M184V is selected by 3TC (lamivudine) or FTC (emtricitabine), leading to high-level phenotypic resistance. Despite this resistance, 3TC and FTC retain some antiviral activity.
- It impairs viral fitness. Viruses with M184V just don’t grow as well in vitro.
- It improves susceptibility to tenofovir, zidovudine, stavudine. By contrast, it worsens susceptibility to abacavir and didanosine.
Part 1 also mentioned that, despite how common this mutation was in clinical practice, we still didn’t have clarity about the best treatment option with this mutation. The date of that post was May 21st, 2017, and here’s the good news — there’s been quite a bit of progress on this front.
The most important data come from the DAWNING study, which evaluated people who had failed first-line treatment with two NRTIs and an NNRTI, then randomized them to receive either dolutegravir or lopinavir/ritonavir, plus at least one active NRTI as measured by baseline genotype testing.
The study was an overwhelming win for the dolutegravir strategy (84% vs. 70% viral suppression in the DTG vs. LPV/r arms, respectively), prompting early termination of the trial. The results certainly made us much more comfortable using dolutegravir plus NRTIs as a second-line treatment option, even in patients harboring some NRTI resistance.
Not surprisingly, most participants (84% in DTG arm, 81% in LPV/r arm) had M184V at baseline, which if present did not alter the primary study results favoring DTG. Most of the subsequent NRTI regimens chosen included either TDF or ZDV.
(Time for a complex, kind of geeky, digression. DAWNING let the investigators choose what NRTIs they used to combine with DTG or LPV/r. In the WHO guidelines, which assumed no genotypes available, and hence possible K65R, people previously receiving TDF were advised to go on ZDV, and the converse. As a result, it is likely that in DAWNING some of the participants who had M184V alone went on ZDV, even though they didn’t “need” to — tenofovir would have been active. And we hate to use ZDV these days, don’t we? Plus, two people in the study did have subsequent treatment failure with integrase resistance — but none of them had M184V alone.)
So, DAWNING takes care of people who are failing therapy with isolated M184V — the best approach is to give them DTG with at least one active NRTI, with tenofovir the ideal choice, given its enhanced activity (see Point #5, above). Often we’ll keep the FTC (or 3TC) along for the ride, since it’s so nontoxic and, as noted in Point #3, still has some activity.
But what about suppressed patients? What if they have M184V, and you’re interested in switching?
Here we can get some information from the 4030 study, presented this year at IAS. (I was the presenting investigator.) People with viral suppression on DTG plus TAF or TDF/FTC were randomized to TAF/FTC + DTG or to switch to the single pill BIC/FTC/TAF. Unlike most switch studies, prior failure with resistance was permitted, and participants were stratified by NRTI resistance into three groups:
- Tenofovir resistance (either K65R or 3 or more TAMS)
- Any other NRTI pattern (which includes M184V, either alone or with others)
- No NRTI resistance
The overall results demonstrated noninferiority of BIC/FTC/TAF to DTG plus TAF/FTC. These results held up regardless of the baseline resistance category.
Ah, but what about those challenging patients with both tenofovir resistance plus M184v? (This is the and More! part of the title.)
Time for another email, this time from another ID/HIV specialist — Dr. Risa Hoffman, from UCLA!
Hi Paul,
In the study you presented at IAS – did any people have both K65R and M184 (or in other words, resistance to both TAF and FTC/3TC?). We are doing a journal club on the ever-favorite topic of using bictegravir/FTC/TAF or a similar regimen in the presence of NRTI resistance…. I’m still looking for the answer about using this regimen when someone has mutations to both the TAF/TDF and FTC (I think you posed this question to the DAWNING study team at CROI…). Seems risky.
Thanks!
Risa
This is the kind of detail one can’t put in a 10-minute conference presentation, so here it is:
Of the 30 participants in that first category (which is resistance to tenofovir by either K65R or lots of TAMS), 21 also had M184V/I (13 BIC, 8 DTG). Of these 21 participants, 4 had K65R with M184V (3 BIC, 1 DTG), and the rest (17) had TAMs with M184V (10 BIC, 7 DTG).
And none in either arm had treatment failure at week 48.
So where does that leave us?
In the setting of treatment failure, I’m quite comfortable that patients with M184V alone can be successfully treated with tenofovir (TAF or TDF)/FTC plus DTG. BIC/FTC/TAF would probably work too, though it hasn’t been tested.
For people who are already suppressed on an integrase-based or NNRTI-based regimen, they can be successfully managed with either DTG plus tenofovir/FTC or BIC/FTC/TAF, even if they have more than just M184V for NRTI resistance. There is enough residual activity of the NRTIs to let the high resistance barrier integrase inhibitors (DTG or BIC) do most of the work.
Two key remaining questions:
- For treatment failure with K65R plus M184V, what is the best approach? ZDV is highly active against these viruses, but who wants to use it? Should it be DTG plus DRV/r? Something else?
- For suppressed patients on NRTIs plus a boosted PI (and there are a lot of them in the world), can they be switched to DTG plus TAF/FTC or BIC/FTC/TAF? Even if we don’t know their resistance history?
When clinical research answers those questions, it will be time for The Curious Case of M184V, Part 3.
Hey, it’s still officially summer, at least until September 23 — let’s go swimming!
September 2nd, 2019
New Antibiotics for CRE, Draft Lyme Guidelines, Cost of Measles Outbreaks, and More — a Labor Day ID Link-o-Rama
 Labor Day! Could summer really be over?
Labor Day! Could summer really be over?
Nah, we still have a few weeks — and as I’ve noted several times, this time of year (September-October) gives us far and away Boston’s best weather season.
On with the links.
- Data from electronic medical records can accurately identify the best candidates for PrEP. The challenge in primary care is finding the tiny proportion of a clinical practice that could benefit from PrEP — and here’s a way to have the medical record do it for you. This is very nice work here, with some controversy (apparently some might find this creepy). And note that this algorithm only worked in men.
- PCV13 may no longer be recommended to all immunocompetent older adults. Instead, “shared clinical decision-making” will likely become the guideline, based on the plummeting rate of invasive disease in those over 65 due to pneumococcal immunization of children. Great coverage here by Dr. Abigail Zuger, right here in our own NEJM Journal Watch. But big question — how best to engage in this process, a patient-driven strategy I suspect we’ll see increasingly in all sorts of guidelines going forward. And will it influence insurance coverage for the vaccine?
- Uptake of the new anti-CRE drugs — for treatment of CRE — is surprisingly slow. The previous standard of care (usually polymixins) is terrible, so what’s up? The probable explanation is a combination of unfamiliarity and (especially) high cost — especially since these are hospitalized patients.
- Here’s another study showing no clinical benefits to HIV regimens with high CNS penetration effectiveness (CPE) scores. Could it be time to abandon this as a desirable metric for HIV treatment? Except in truly unusual circumstances — so called “CNS escape” cases — suppression of HIV replication in the central nervous system tracks quite nicely with activity in peripheral blood.
- IDSA, the American Academy of Neurology, and American College of Rheumatology issued draft guidelines for diagnosis and treatment of Lyme Disease. Public commenting will close September 9 — final publication eagerly awaited!
- Chilling summary how Lyme can become an “identity.” Note that the author of this well-researched, difficult, but very fascinating piece never denies the existence of post-Lyme disease syndrome (which is very real), yet is accused of doing so in the comments. I suggest every ID doctor read both this and this account in The Atlantic, from a sufferer, who is just trying to get better, and doesn’t know what to believe.
- The Truvada lawsuit may be hindering PrEP uptake. People notice the ads soliciting cases from personal-injury legal firms — both patients on HIV treatment (including several of my own, who have asked about it), and individuals considering PrEP. Here’s a sample, on autoaccident.com (of course, where else). Hard to imagine this will be a good thing for anyone.
- People with perinatal HIV transitioning to adult care have high rates of treatment failure. In a time when essentially all people with HIV in care have suppressed viral loads, the fact that 56% of this cohort transitioning to adult care had viremia stands out starkly — and underscores how challenging care for this population can be.
- Even small measles outbreaks cost public departments a lot. Infuriating, because these government funded programs are not exactly swimming in money, or just sitting around waiting for other things to do. This outbreak in a Clark County, WA cost nearly a million dollars; the one in New York is pushing 20 million. If only there were a solution to the problem of measles … oh wait.
- This New Yorker piece summarizes some of the origins of the anti-science views fueling the anti-vaccine movement. It’s exceptionally well-written — I particularly like this tally: “Suspicion of authority, rejection of expertise, a fracturing of factual consensus, the old question of individual liberty versus the common good, the checkered history of medical experimentation (see: Tuskegee, Henrietta Lacks, Mengele), the cynicism of the pharmaceutical industry, the periodic laxity of its regulators, the overriding power of parental love, the worry and suggestibility it engenders, and the media, both old and new, that feed on it—there are a host of factors and trends that have encouraged the spread of anti-vaccination sentiment.” As for the media, I can’t resist posting this brilliant cartoon:
In the interest of balance… pic.twitter.com/pDLAzTPz5q
— TwistedDoodles (@twisteddoodles) August 22, 2019
(H/T to Keith Law for finding the cartoon.)
- HIV therapy and CRISPR-Cas9 eradicate HIV. In mice. Humanized mice, but still, mice. Have to start somewhere!
- With this recently approved Lyme antibody test, can we eliminate the need for the Western blot as a confirmatory test? The strategy would involve doing two immunoassays instead. Every ID clinician has experienced both the delay in diagnosis by waiting for the Western blot, as well as the rampant confusion over the results — especially with IgM results.
- Complex HIV regimens can be simplified even if there’s extensive background resistance. This certainly is our practice, but caution — you need complete resistance information! Plus, best to do close follow-up after making the switch, if for nothing else to reassure your patients who may be understandably fearful that fewer drugs will lead to treatment failure.
- Should serum β-D- glucan testing be used for the diagnosis of Pneumocystis jiroveci pneumonia? Two papers take opposite sides on this question, including one led by our former fellow (and now ID attending at Maine Medical Center) Dr. Jed Pilkington. (I was co-author.) I won’t divulge what side we took, but here’s the bottom line: The test is great if ordered in the right clinical context — but too often that’s not the case, and the results are frequently impossible to interpret!
- The number of reported cases of emergent dolutegravir resistance when part of an initial triple-therapy regimen is now up to 3. All cases involved very high baseline viral loads and low CD4 cell counts — 1,970,000 and 78 in the first case, 457,000 and 39 in the second, and 1,400,000 and 22 in this third one. Two also received rifamycin drugs. As noted in the title to the second case report, it’s time to conclude that selection of DTG resistance in initial therapy is “rare but possible.”
To finish — slow and steady wins the race (at least sometimes). I’ve watched this video too many times not to share it on this site, as it’s strangely compelling.
August 25th, 2019
Save the Dates! International Scientific Conferences You Can’t Miss
 On this beautiful summer Sunday, I’m sorting through a series of invitations to international scientific meetings.
On this beautiful summer Sunday, I’m sorting through a series of invitations to international scientific meetings.
So many options.
See, they really want me. And how do I know that my participation will be so important?
Just look how personalized the greetings are — clearly intended for me and me alone. I’ve included actual screen shots of the invitations as evidence for the careful thought that went into sending me — yes, me — these invitations.
To say I’m flattered barely begins to describe how these emails make me feel.
First up, from an “Auston Matthews”, who describes himself (herself?) as “Program Manager”:
Dear Mr./Ms. Matthews,
I’m very well indeed! And how are you? How are you enjoying the role of Program Manager?
Now that we’re done with the niceties, let me assure you — the privilege and honour are 100% mine. Being a Speaker towards the 3rd Global Experts Meeting on Infectious Diseases would be something special. I’ll highlight it on my CV in bright yellow marker. Please relay that (plus my best wishes) to the Organizing Committee Members, who no doubt are busy in London planning an exciting meeting.
Before I make my flight arrangements, where is “Bankok”? Is that near Bangkok?
With warm regards,
Dr. PE Paul E
Next — an email from a certain “Henry Walter, Conference Manager”, who generously offers me the chance to visit Rome!
Academic medicine, what a global endeavor!
My response:
Dear Henry Walter,
May I assume we both can communicate on a first-name basis, since you used mine? Hope that’s not too presumptuous!
And you bet I’ll participate in that conference! Not only were the first three iterations of the International Conference on Neurology and Brain Disorders (INBC) scintillating, but I’ve heard that the 4th — INBC 2020 — will absolutely rock.
Let’s speak ASAP about my topic, as I have some ideas. How about a lecture entitled, “Degenerative Brain Diseases of Fish”? After all, I’m the expert.
Hope this sounds INBC-worthy to you. I know the standards are very high!
Sincerely,
Default Value
On to invitation number three, which represents the very pinnacle of personalized conference invitations. It comes from a “Dr. Irene Frank”, whose title is “Executive Director, Coalesce Research Group”. They’re based in Greenville, South Carolina, in case you were wondering.
Here’s what I wrote back:
Dear Dr. Frank,
First, very warm wishes back to you! Speaking of, it must be hot this time of year down in South Carolina, ha ha.
Second — Plenary Speaker at Nano 2019, what an honor! I’m speechless. But don’t worry, I’ll get over it by November, and will be ready to go for my plenary talk. I’ll make sure it’s something BIG about this small (nano, get it?) topic. Ha ha again.
Third — just curious, is it hard to organize a conference in Osaka all the way from Greenville, South Carolina? Is there a direct flight? It’s amazing how you folks at Coalesce Research do all that you do, so impressive.
Finally, before formally agreeing to speak, I have to ask — how will you be introducing me? It’s just not clear from your invitation. And I confess — sometimes I feel like a nowhere man, sitting in a nowhere land, making all these nowhere plans, for nobody.
Regards,
“,”
Take it away, guys.
(Part of an occasional series, as these folks show no signs of tiring. And h/t to friend John Winkelman for the inspiration.)
August 18th, 2019
Choosing the Top Research Papers in HIV Medicine — and Recalling the Joy of Working with a Great ID Fellow
 Way back in 2008 — the year I started writing here — I drafted an exceedingly long post listing the top published papers in HIV medicine.
Way back in 2008 — the year I started writing here — I drafted an exceedingly long post listing the top published papers in HIV medicine.
Oh how I tortured myself over that thing.
How to define “Top”? Most cited? Most clinically important? Most rigorously scientific? Best written?
After a while, I just abandoned the monstrosity. It still sits electronically somewhere in the NEJM Journal Watch servers, forlornly awaiting a revision.
Now, courtesy of my friend and colleague Dr. Raphael (Raphy) Landovitz from UCLA, the concept lives again. He joined me for the Open Forum Infectious Diseases (that’s “O-F-I-D”, not “Oh-fid”) podcast, where we each choose our top 5 research papers in HIV medicine — plus our least favorite.
Give it a listen, or read the transcript. We’re also on iTunes, where we’d appreciate a rating if you like what you hear.
Before leaving you, just a few words about Raphy and how great he was as an ID fellow. Since we’re ranking things, he’s easily among the top ID fellows I’ve ever worked with in my gazillion years of being an ID attending. Energetic, smart, compassionate, thorough — ticked all the boxes. He also was hysterically funny.
(Brief aside — working with great fellows is a true joy. On service or in clinic with them, you totally trust their clinical instincts, and end up finishing each other’s sentences while discussing a case. A wonderful synergy in academic medicine.)
After finishing his fellowship plus a few years on the faculty here, Raphy traded our cold Boston winters for the warmth (but geologic instability!) of Los Angeles. Under the the skillful mentorship of Dr. Judy Currier, he’s had a sensational research trajectory, focusing on HIV prevention. There is not a person on the planet who knows more about PrEP. And, just this month, Raphy was deservedly promoted to full Professor of Medicine at UCLA.
Hoping you’ll permit those of us involved in some way with his training to beam a little!
August 11th, 2019
The United States Needs Stricter Gun Control Now — and Yes, This Is an ID Issue
 In general, I’ve tried to keep this site a pretty happy place.
In general, I’ve tried to keep this site a pretty happy place.
It’s not been difficult. The ID and HIV community includes many smart, like-minded individuals involved in all sorts of interesting and challenging work, both domestically and abroad. As one of our ID fellows recalled, after he did a rotation in our ID clinic during his medical residency, “I realized I’d found my people.”
Today, however, I’m going to write about something on the sadder side, an issue tearing up our country.
We need stricter gun control in the United States. And we need it NOW.
Yes, this is a departure from prior controversies here, such as should doctors wear white coats, estimating the infectious risk of food tongs, or whether the correct abbreviation for P. jirovecii pneumonia is now PJP instead of PCP.
I’m venturing into this more contentious* issue because it’s just too painful to ignore.
(*But to my dear readers of the ID persuasion, there’s an excellent chance you’re in complete agreement. And how do I know? Easy.)
How many of you thought, Oh no not again, when you heard the news of last week’s shooting in El Paso? And then less than 24 hours later, Oh no not again, with the news from Dayton?
And we could go on and on listing these mass shootings — sadly, we even have a Wikipedia page cataloguing them for us. It’s getting so bad that voices outside of the usual crowd feel compelled to weigh in — here’s sportswriter Joe Posnanski taking a break from his usual (brilliant) baseball coverage to write a poem describing his heartbreak.
It’s very moving. But a sportswriter writing a poem? Must be time to do something.
And lest some think this isn’t a problem related to Infectious Diseases, that I should “stay in my lane” — how wrong to think that. It’s a problem for all of us in healthcare, and, for several reasons, especially for us ID-specialists.
Crystal Zheng and David Mushatt, two ID doctors from New Orleans, astutely make the case:
During the initial hospitalization, gunshot wounds can become infected, causing skin and soft tissue infections, as well as intra-abdominal, thoracic, and brain abscesses ….Long after the initial event, gunshot survivors with spinal cord injury face a lifetime of disability, marked by recurrent infections due to autonomic dysfunction, decreased airway clearance, sensory loss, and paraplegia. These patients are at increased risk for urinary tract infections, pneumonias, infected sacral ulcers, and chronic osteomyelitis. Infection is the leading cause of death in this population.
(Emphasis mine.)
Most of the cases we see, of course, aren’t from mass shootings. Events like El Paso and Dayton (and Gilroy and Newtown and Orlando and Parkland and Charlotte and Pittsburgh and …) get the big news, but there are way more injuries and deaths from individual shotgun wounds happening every day and night somewhere in our country.
For the victim, it doesn’t matter if the injury is from a mass shooting, an unfortunate urban skirmish, a heated domestic squabble, or the result of a deranged individual who has ready access to a gun. Their lives are irrevocably changed if they survive, often with infectious complications.
Yes, if they survive. If they don’t, those left behind suffer unbearable sadness and trauma at the loss — family, friends, colleagues. We have personal recent experience at our hospital when, in 2015, one of our rising stars in cardiac surgery was shot during an outpatient clinic session.
Oh no not again.
But look — it doesn’t have to be this way.
After last weekend’s events, I tweeted about the New Orleans perspective piece cited above, and received this response:
I’ve not seen gun shot wounds in Australia, despite 35+yrs of clinical ID practice. But I looked after the Bali bombing burns/multi-trauma victims that were evacuated to Perth, in Australia. I’ve also seen every other type of multi-trauma & injuries that I can think of…
— Christopher Heath (@Christo37020634) August 7, 2019
That’s right — 35 years of clinical ID practice, no gunshot wounds. Other countries have angry people. Other countries have urban violence. Other countries have terrorists. But only in the United States can these lead to such efficient killing.
And it’s the easy access to guns that makes our country an outlier in gun violence. It’s that simple:
In 2017, we looked at possible explanations for why the U.S. has so many mass shootings. This is what we found. https://t.co/AzcBigwEaw
— The New York Times (@nytimes) August 3, 2019
I hope you’ll read the linked article, which debunks several popular alternative theories about why we have more gun violence (specifically mass shootings) than any other country. But if you don’t have the time:
Americans make up about 4.4 percent of the global population but own 42 percent of the world’s guns. From 1966 to 2012, 31 percent of the gunmen in mass shootings worldwide were American.
New Yorker writer Adam Gopnik has written frequently on this topic, perhaps because he lived in France, a place with far more sensible gun control. After the Orlando mass shooting in 2016, he wrote a piece called “One Person, One Gun” highlighting the horrible power we Americans grant our citizens — criminals, terrorists, people with mental illness, perpetrators of domestic violence — by giving them ready access to guns.
And it’s not just any handguns, but weapons designed for military use specifically to kill as many people as efficiently as possible:
So, yes, one person did do that, but it was a weapon that empowered him to do it—a weapon designed only for mass killing on the battlefield, a weapon so dangerous that soldiers keep their version locked up when not actually training with it, out of respect for its rapid-fire lethality, but a weapon that now, in Florida and elsewhere, can be placed freely and without constraints into the hands of almost anyone who wants one.
Last week, numerous medical organizations weighed in on the need for stricter gun control in this country, calling for specific actions that make so much sense it only adds to the pain that these restrictions are not already in place — among them, requiring background checks before sales, prohibiting gun ownership among those guilty of domestic violence, special regulation of high-capacity firearms, and removal of physician gag-orders on counseling about gun violence.
I can’t speak on behalf of the Infectious Diseases Society of America (our own medical society), even though I’m a member.
But I can unequivocally say that 100% of the ID clinicians I’ve spoken with agree that this is an urgent issue for all of us.
Take it away, Stephen. It may not be your funniest clip on the topic of gun control — search YouTube for “Stephen Colbert gun control”, there are plenty.
But it could be your most important.
July 28th, 2019
Really Rapid Review — IAS 2019 Mexico City
As I noted last week — and you did read last week’s post, didn’t you? — the International AIDS conference first took place in Mexico City in 2008. Last week we returned to this sprawling, vibrant city for the 2019 meeting.
It was an excellent, well-run conference — with one small complaint.
But more on that later… On to the content, a Really Rapid Review® of the highlights.
- The risk for neural-tube defects in babies born to women who conceived while receiving dolutegravir is lower than first estimated. In last summer’s report from Botswana, it was 4 out of 426 (0.9%); now with a larger surveillance population (covering over 70% of nationwide pregnancies in that country), it was 5 (one additional case since the original four) in 1684, or 0.3%. This is still higher than in women taking EFV at pregnancy, or women without HIV (around 0.1%), but good news overall. So the relative risk is still higher, but the absolute risk is low. (Linked is the full paper, published when the study was presented.)
- Additional cohorts from Botswana and Brazil also demonstrated a lower risk than the original estimate. The cumulative effect of these three studies enabled the WHO to go forward and recommend “TLD” — tenofovir, lamivudine, dolutegravir — to all people starting HIV treatment, even women of childbearing potential. Bottom line — we clearly should not be switching all women of childbearing potential off their dolutegravir-based regimens. But what if a woman says she wants to become pregnant, and is actively trying? Then we’ll need to have a discussion, and I believe her preference should dictate what we do.
- Could PrEP with TAF/FTC work faster and be more forgiving of poor adherence than TDF/FTC? In this sub-analysis of incident HIV from the DISCOVER study comparing the two, poor adherence was responsible for breakthroughs in both treatment arms. But the onset to protective concentrations was faster for TAF than TDF, and duration of protection after the last dose of tenofovir was 60% longer as well. TAF/FTC is currently under review by the FDA for PrEP; for now, I would recommend its use in people who have some clinical reason for not taking TDF — such as known renal disease, low bone density, or perhaps during the time of bone formation (adolescents and young adults).
- Raltegravir is not quite as good as efavirenz as initial therapy for people with HIV who have active TB. Or, in the pedantic words of clinical trialists (i.e., people like me), it was “not noninferior.” Another finding — no increased risk for IRIS with the RAL-based approach. So efavirenz will remain standard-of-care for now, but given our general move to second-generation integrase inhibitors, how would efavirenz compare with twice-daily dolutegravir?
- Dolutegravir/lamivudine remains noninferior to dolutegravir plus TDF/FTC at 96 weeks. The key finding from this extended follow-up of the GEMINI (as in the twins, two drugs, ha!) study is that still no participant developed resistance in the two-drug arm. It will be interesting (and important) to see if this resistance barrier is just as durable in clinical practice, where adherence tends to be lower than in clinical trials.
- In a large US cohort study (n=2038), DTG-based regimens achieved a higher rate of viral suppression than any of EVG, RAL, or DRV. This certainly would reflect current clinical practice for these patients, though some would use bictegravir/FTC/TAF (which is very similar to DTG + 2 NRTIs). An ongoing prospective study (cleverly called LAPTOP) compares bictegravir/FTC/TAF to
darunavir/c/FTC/TAF in patients with advanced HIV disease. - A randomized, three-armed study in South Africa compared TDF/FTC/EFV, TDF/FTC plus DTG, and TAF/FTC plus DTG. Results — noninferior virologic efficacy across the three arms, and a bit more failure with resistance in the EFV arm. Two important additional comments: 1) The study was over 60% women, differentiating it from USA/European studies, which are overwhelmingly in men; 2) Weight gain was significantly different per study arm. The DTG regimens gained more weight than the EFV regimen, and the TAF/FTC plus DTG regimen gained the most weight (10 kg at 96 weeks in women). (Link to the published paper, published as the study was being presented.)
- A focused analysis on weight changes in the ADVANCE (previous link) and NAMSAL studies confirms that initial therapy with DTG leads to more weight gain than EFV. The NAMSAL trial compared DTG with low-dose EFV in Cameroon, with a very advanced HIV disease population and again more than 60% women. The cause of excessive weight gain associated with certain ART (in particular DTG and TAF) remains unclear, but it is certainly more than just a “return to health” — these changes shift more people into the overweight or obese categories. The big question is why? And why more in women — especially black women — than men? Leading theories include a possible appetite-suppressive effect of TDF and/or a still undefined off-target toxicity of integrase inhibitors, or TAF, or both.
- Bictegravir/FTC/TAF is safe and effective in women. Regulatory studies of this commonly used single pill treatment did not include many women, so data from this study of over 400 women are important. Analysis of weight changes are ongoing.
- Switching people on TAF-based, three-drug ART to dolutegravir/lamivudine is noninferior to continued triple therapy. This study — called TANGO, because it takes two (ha) — further demonstrated a very low risk for treatment failure and no emergent resistance in either treatment arm. Importantly, eligibility criteria excluded participants with a history of treatment failure or resistance — so people who have never failed are the appropriate target of this regimen switch.
- For people on TAF/FTC or TDF/FTC plus dolutegravir, switching to bictegravir/FTC/TAF was noninferior to TAF/FTC plus dolutegravir. (Disclosure: I was the presenting author.) To contrast with the prior study, here participants were eligible even with prior treatment failure and/or resistance — and approximately 25% did have NRTI resistance, as evidenced by either investigator-provided genotypes or an assessment of proviral DNA (“archive”) resistance testing. The study results suggest that the resistance barrier of bictegravir is comparable to dolutegravir, as it was able to maintain viral suppression even with NRTI resistance.
- The two-drug regimen of DTG plus DRV/r maintains virologic suppression as well as continued three-drugs. This strategy should perhaps be called a three-drug regimen (two active drugs) since the DRV needs to be boosted with either ritonavir (as in this study) or cobicistat.
- Patient-reported outcomes with long-acting injectable cabotegravir plus rilpivirine demonstrate very high rates of treatment satisfaction. With the caveat that those entering this study of oral vs. injectable therapy knew they had a 50% chance of getting the latter — and hence that’s probably why they entered the study — these data show that this first version of injectable therapy will be highly popular among those who want it. FDA approval likely in early-mid 2020.
- An implant of the antiviral agent MK-8591 (islatravir, ISL) provided sustained drug levels that could be sufficient for PrEP for up to 1 year. Yes, this meeting was the first time we’d learned the name of the potent nucleoside reverse transcriptase translocation inhibitor we’ve been hearing about for a few years. The same company makes an FDA-approved long-acting contraceptive (Nexplanon), which should hasten development of this novel approach to prevention.
- Islatravir, doravirine, and lamivudine demonstrated comparable virologic suppression to a control arm of tenofovir DF/3TC/doravirine. A dose-finding phase 2 study, so a sample size small (roughly 30 per arm). The goal will be to move this to a two-drug regimen of daily islatravir/doravirine — though clearly the former could be administered less frequently, provided it finds an appropriate dance partner.
- The attachment inhibitor fostemsavir induced viral suppression at 96 weeks in 60% of people with only one other active drug. Importantly, most participants received dolutegravir (fully or partially active), and a multi-drug optimized background regimen (OBR). Perhaps even more remarkably, 37% of a smaller group with no active drugs (but still given with an OBR) achieved viral suppression. Fostemsavir has not yet been submitted to the FDA for review, where the intent is to seek approval as a therapy for people like those in this study — with limited treatment options.
- A single subcutaneous dose of the capsid inhibitor GS-6207 induced a viral load decline of 1.8 to 2.2 log at day 10. At that point in the study, all started BIC/FTC/TAF. This agent could be part of a once-weekly or even much less frequently administered regimen; it also may have a role in people with limited treatment options. Except …
- There aren’t many people left in the USA who have limited treatment options. As I wrote last week, this has become increasingly rare — <1% of people in care, according to this comprehensive evaluation of over 27,000 ART-experienced people with HIV in the USA. Finding those who are also viremic and eligible for clinical trials of novel agents will be a challenge!
- In low-to-moderate cardiovascular risk individuals with HIV, rosuvastatin did not slow progression of atherosclerosis as estimated by carotid artery intima-media thickness. These negative results are relevant for the much larger REPRIEVE study, which is testing pitavastatin in a similar population, but using clinical endpoints.
One big plus to this year’s conference — the number of research posters was smaller than usual, increasing the quality of accepted studies.
Also, being in Mexico City in the summer remains a joy, with cool weather (remember the altitude!), friendly people, extraordinary sites and cuisine, and relatively reasonable prices.
My only complaint? No, not the traffic — that’s terrible in every big city.
It was the conference Wi-Fi, which tortured every attendee in a particularly excruciating way. Your computer and/or phone would indicate everything A-OK, no problem, then give access to the internet in brief spurts, lasting at most seconds — followed by prolonged periods of unresponsiveness. Completely impossible to do anything.
WiFi at #IAS2019 worse than early versions of WiFi on flights. Yes, #FirstWorldProblems, but still plenty frustrating!
(p.s. great conference otherwise.) https://t.co/G6fxaWkGPj— Paul Sax (@PaulSaxMD) July 24, 2019
Yes, I’ll get over it.
July 21st, 2019
AIDS Conference Returns to Mexico City, Where We Saw an Underrated, Great Advance in HIV Therapy
If you’ve been an ID or HIV specialist for only a decade or so, the following statement might seem unfathomable to you:
Until 2008, there were lots of people with HIV whose medication adherence was perfect — but they still had virologic failure.
How could that be? The simple answer is that their virus had too much resistance to the then-available drugs.
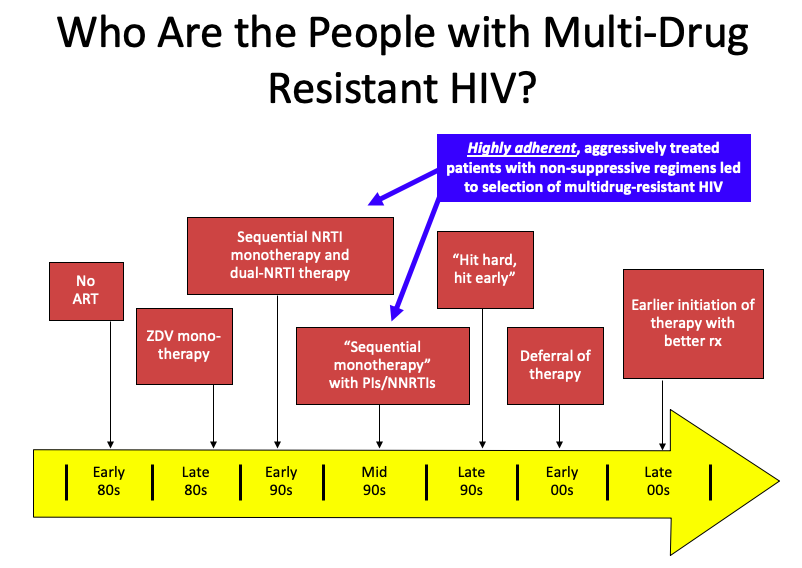
But it’s a bit more complex than that. Several factors converged, summarized in this PowerPoint slide:
(I once upon a time thought this slide originated with Steve Deeks — but now he denies creating it, so I’ll take “credit”. Hey, good slides are like recipes, incrementally adapted until they become your own. If the originator wants to come forward, I’m happy to add your name to the slide.)
It’s those people in the blue box that have the most extreme multi-class HIV drug resistance. Treated in the early-1990s with single or dual nucleoside reverse transcriptase inhibitors (NRTIs), they already had NRTI resistance when the protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) because available in the mid-1990s.
Addition of a single active drug from these classes, often of marginal potency (nevirapine, delavirdine), or suboptimal pharmacokinetics (saquinavir, nelfinavir), or toxicity (ritonavir) acted as “sequential monotherapy”.
What we know now is that if they didn’t also add lamivudine as a new drug (which was still very active against most NRTI-resistant virus at the time), they were likely to experience treatment failure, with resistance to yet another drug class.
It didn’t help that we didn’t fully understand within-class resistance — for example, it was believed that the resistance profiles of zidovudine and stavudine were sufficiently different to transition a patient from one of these drugs to the other. Ditto certain nevirapine mutations and efavirenz. Not true!
The fact that our treatments failed in some of our most devoted and adherent patients resonated strongly with us HIV specialists. It was heartbreaking. Some had been hopeful volunteers in early clinical trials, which exposed them to inadequately dosed single-drugs that then led to a loss of this drug class. In hindsight, how ironic — and how tragic.
Others had to go through desperate (but ultimately failed) attempts to achieve viral suppression, including:
- “Mega-HAART” — using 6 or more drugs in untested combinations.
- “Double-boosted PIs” — two protease inhibitors with ritonavir.
- Hydroxyurea — raised intracellular concentrations of didanosine, with many untoward effects. Ugh, even writing that we prescribed that combination makes me cringe.
- Treatment interruptions in order to “resensitize” their virus to the drugs we did have — didn’t work, led to increased risk of disease progression.
The silver lining to this resistance was that the virus became less fit; the viral load rebounded, but there was a substantial delay before the CD4 cell count declined. Patients remained remarkably stable clinically even with viremia and resistance.
This disconnect between viral load rebound and CD4 decline allowed many to survive until the miracle year of 2008, when finally we had a potent new drug in a new drug class — raltegravir, the first integrase inhibitor, approved in 2007 — plus drugs from existing classes with activity against most resistant viruses, specifically darunavir (PI, approved in 2006) and etravirine (NNRTI, approved in 2008). (Maraviroc played a less important role, since most of these patients already had non-R5 virus.)
Why bring this up now? On the eve of the 2019 International AIDS Conference, which takes place this week in Mexico City, I think back to when the conference was here last — that magic year of 2008.
It was during that 2008 Mexico City meeting that we first saw data from the TRIO study (later published here, lead by Yazdan Yazdanpanah), which enrolled participants with resistance to NRTIs, NNRTIs, and PIs, and gave them all three of these new drugs — raltegravir, darunavir, and etravirine.
The results were staggeringly good — 91% had viral suppression at week 24, a rate quite comparable to responses in treatment-naive patients with no resistance. It was actually a bit better, reflecting the high level of adherence for these motivated participants. In HIV clinics around the world, this “TRIO” regimen became the default standard for treating multidrug-resistant HIV — and it worked just as well in clinical practice as it did in the clinical trial.
At last, we could effectively treat these brave people who had weathered years of viral failure, immunosuppression, and the toxicities of early antiretroviral therapy. Many experienced the joys of hearing they had an undetectable viral load for the very first time. Treatment failure with multi-class resistance became increasingly rare — so much so that a popular HIV drug resistance test stopped selling its product, the laboratory equivalent of closing an inpatient hospice for people with AIDS.
I’m happy to report that this wasn’t a brief honeymoon phase — responses were durable, and most people with multidrug-resistant HIV have had viral suppression since 2008. The reality is that in many parts of the world, the patient with “no treatment options” due to resistance has become an exceedingly rare occurrence.
Even busy practices and referral centers see very few of these cases; in the past two years, most have seen zero:
Hey HIV treaters out there–in the past 2 years, do you follow, or have you seen, any people with viral failure and resistance to ALL major HIV drug classes? (Enfuvirtide and ibalizumab excluded.) If yes, share how many in the replies. (I've seen 2.)
— Paul Sax (@PaulSaxMD) July 20, 2019
This is not to say that it never happens anymore — a paper published recently in Clinical Infectious Diseases enrolled 20 such individuals, admitting them to an NIH inpatient center for directly-observed therapy. Of note, in this group (which included several with perinatal HIV), nonadherence turned out to be the primary reason for failure in 9 of the 20.
So here’s to 2008, a year when our knottiest HIV-resistance problems became solvable — hooray! It was a tremendous advance, one underrated in my opinion when people retell the history of antiretroviral therapy.
And what we will learn this year in Mexico City? That will be next week’s post!
Speaking of drug resistance, here’s another take:
July 14th, 2019
The House of God Profiled Physician Burnout Long Before We Called It That — Should Aspiring Doctors Still Read It?
Many consider the novel The House of God, written by Samuel Shem (pen name for Stephen Bergman), to be a must-read for any physician or soon-to-be physician. A fictionalized account of his internship year, the book details how the accumulated stress, fatigue, and powerlessness of being a first-year doctor inexorably accumulates during that year — with sometimes hilarious, and but also disastrous, results.
The survival strategies of the interns in the book under such circumstances are not pretty. Cynicism becomes the default attitude, an us-versus-them camaraderie that considers the “them” to be not only hospital administrators and older staff physicians — but painfully, the patients, too.
Other survival tactics fall into the “instant gratification” category — the book is packed with sex, and drinking (but especially sex) — which no doubt boosted its readership, and also (warning) makes re-reading it today somewhat cringe-worthy, I imagine especially for women.
This insider look delivered by The House of God was at first widely criticized by the medical establishment. However, over time, the book has become a canonical and widely praised real-world depiction of internship — many doctors I know have read it, and it even appears in the humanistic curricula of some medical schools.
I first read the book in college, a time when I was seriously considering medical school. I found it funny, and surprising — could young doctors really be having so much sex? — but also quite sad (one of the characters takes his own life). And the grotesque depiction of the patients, especially the elderly and infirm, made me very uncomfortable, an undoubtedly intentional goal of the author to show how depersonalizing the whole experience had been.
Frankly, it filled me with dread about the prospects of becoming a doctor.
Re-reading it later, some years after my own training, I saw the extreme perspective as a necessary cry for help — these interns clearly manifested all the characteristics we now commonly refer to as physician burnout.
I could also appreciate the clinical insights embedded in the famous “Laws of the House of God”— #3 is absolutely brilliant:
3. At a cardiac arrest, the first procedure is to take your own pulse.
So my re-read found nuggets that included a human side to doctoring — The Fat Man character is clearly a great clinician — albeit these are few and far between. It’s no wonder that the online reader reviews of this book are so disparate, with plenty of angry one-star reviews mixed in with the general praise.
So why is it in the news now? And why bring it up here?
JAMA this week published a thoughtful perspective on The House of God, plus a piece by Bergman himself. It’s not just to mark the 40th anniversary of its publication; Bergman plans to release a sequel in November. Entitled Man’s 4th Best Hospital, the book will attack the current focus of healthcare on money, and screens, in particular electronic medical records, targets deserving of satire.
From a “pre-review” by WBUR’s Carey Goldberg:
But more than 40 years on, author Samuel Shem trains the satirical sights of his sequel on two particular targets: screens and money. And he connects them, pointing out that the electronic medical record systems that drive many doctors (and patients) crazy are really fundamentally billing systems pretending to be care systems.
With one of his ultra-simple syllogisms, the Fat Man sums it up:
MONEY KILLS CARE
SCREENS MAKE MONEY
SCREENS KILL CARE
Sounds like it will be another cry for help, this time to get us back to connecting with our patients — to pull us away from our electronic billing (I mean medical) records, and to care for them. I look forward to reading it!
As for why here, on an ID blog, regular readers might have gathered over the years that I have an interest in baseball.
(Hey, understatement sometimes is amusing.)
The House of God reminds me in many ways of another book from that era, the baseball classic Ball Four, another insider look at an extreme work environment — this time playing professional baseball. (And also, for the record, Kitchen Confidential. But that’s a different post.)
And last week, as JAMA released its pieces on The House of God, we baseball fans learned that the author of Ball Four, Jim Bouton, had died.
Just like The House of God, it also appeared in the 1970s, and was similarly widely criticized on release by the establishment — this time, the baseball establishment, who preferred a more white-washed, athlete-as-hero illusion of the players. But despite their protestations, the book was a huge critical success, and is now celebrated as a heartfelt and accurate account of what it’s like to live through the grind of a not-so-successful baseball season.
Also like The House of God, the book is very funny, and the players resort to plenty of sex and drinking (but especially drinking this time) — ways to overcome the stress of incessant performance demands, contract disputes, and relentless travel in a sport where management can dispassionately trade you to another team or drop you to the minor leagues for any decline in productivity.
But Ball Four differs from The House of God in two critical ways. First, it’s an autobiographical account, not fiction — these are real people, and it’s Bouton’s voice we hear telling the story.
Second, and more importantly, the joys of playing the game come through strongly. The many problems Bouton has on that 1969 Seattle Pilots team notwithstanding, he clearly loves baseball and deeply respects the hard-work and talent it takes to succeed at this professional level.
A much-quoted line from Ball Four captures this love perfectly:
A ballplayer spends a good piece of his life gripping a baseball, and in the end it turns out that it was the other way around all the time.
There’s nothing like this in The House of God — no joy from patient care, or their gratitude, or how rewarding it can be to master medical information over the course of residency, and then pay it back by teaching medical students and interns. Sure, there’s camaraderie in the book, but much of it is soured by the brutal experiences they are living through.
One of my colleagues has suggested that people wait until after they complete residency before reading the book.
I think he’s right.
What do you think?