An ongoing dialogue on HIV/AIDS, infectious diseases,
February 12th, 2022
The Rise and Fall of Ivermectin — 1 Year Later

Cheese consumption and death from bedsheets are highly correlated.
Here’s a confession few board-certified ID doctors will make — there was a brief period when I thought ivermectin could very well be an effective treatment for COVID-19.
It wasn’t when the in vitro data first came out. Therapeutic concentrations were not achievable in humans.
Nor when the anecdotal reports started pouring in, and sometimes making news. A former colleague of mine, a smart and clinically active person practicing in the Midwest, contacted me in late 2020 telling me that they acted as the primary medical consultant for a nursing home. Since they started using ivermectin, no patient had died or even been hospitalized from the disease. OK, a hopeful observation, but not proof.
Not when the figures appeared correlating ivermectin use and lower death rates from COVID-19 in some countries, mostly from tropical regions in South America and Asia. These figures always reminded me of these spurious (and often hilarious) correlations. Did you know that per capita cheese production correlated strongly with the number of people who died by becoming tangled in their bedsheets? Who knew? And what’s the mechanism?
And certainly not when these folks started pushing ivermectin with an enthusiasm that is frankly religious in intensity. This group’s treatment “protocols“, with their hodgepodge of antimicrobials (including ivermectin), immunomodulators, and vitamins — the kitchen sink approach — strain credibility.
Nope — my moment of greatest hope for ivermectin came just over a year ago, when Dr. Andrew Hill presented results from a meta-analysis of several randomized controlled trials, a presentation he would later also give to the NIH Guidelines panel. Andrew is a well-respected clinical researcher, someone well-known in the HIV research world.
In his analysis as first presented, the risk-ratio for mortality with ivermectin was 0.17 (95% confidence interval 0.08, 0.35), an 83% reduction in risk for dying. Outcomes for other endpoints (time to viral clearance, time to clinical recovery, duration of hospitalization) also favored treatment over controls.
Hearing these data, I reached out to Andrew to discuss his findings, and he generously discussed them with me. He acknowledged that the data were incomplete, but remained strongly suggestive of clinical benefit. Furthermore, he’d been in direct contact with the researchers who conducted the largest of these studies. They repeatedly reassured him that the data were sound.
I subsequently summarized my thoughts here in a post entitled, “Ivermectin for COVID-19 — Breakthrough Treatment or Hydroxychloroquine Redux?”; writing:
My take-home view? The clinical trials data for ivermectin look stronger than they ever did for hydroxychloroquine, but we’re not quite yet at the “practice changing” level. Results from at least 5 randomized clinical trials are expected soon that might further inform the decision. NIH treatment guidelines still recommend against use of ivermectin for treatment of COVID-19, a recommendation I support pending further data — we shouldn’t have to wait long.
What happened next? I offered Andrew the chance to submit the meta-analysis to Open Forum Infectious Diseases, which after conducting some additional analyses, he kindly did. Remember, at this time in early 2021, we had no readily available effective outpatient treatments COVID-19. Something inexpensive, safe, and widely available certainly would be most welcome.
After peer review and some revisions, with Andrew and his team reducing the survival effect size for ivermectin to 56% (still highly significant) due to some additional studies, we accepted the paper. We simultaneously solicited a thoughtful editorial from my Boston ID colleague Dr. Mark Siedner, entitled “Ivermectin for the Treatment of COVID-19 Disease: Too Good to Pass Up or Too Good to Be True?”
Well, we now know that the second part of Mark’s brilliant title turned out to be the case — too good to be true. Many of the studies on which the meta-analysis was based were highly flawed, and one was outright fraudulent. The fake data problem came to light just shortly after the meta-analysis appeared in print.
To his credit, Andrew promptly contacted me when the news broke. He immediately offered to retract the original paper, and even better to submit a detailed analysis of what went wrong.
This revised paper has just been published, entitled “Ivermectin for COVID-19: Addressing Potential Bias and Medical Fraud.” It includes this extremely telling figure, which shows how the effect size of ivermectin on survival drops to meaningless with exclusion of the fraudulent and potentially flawed studies:
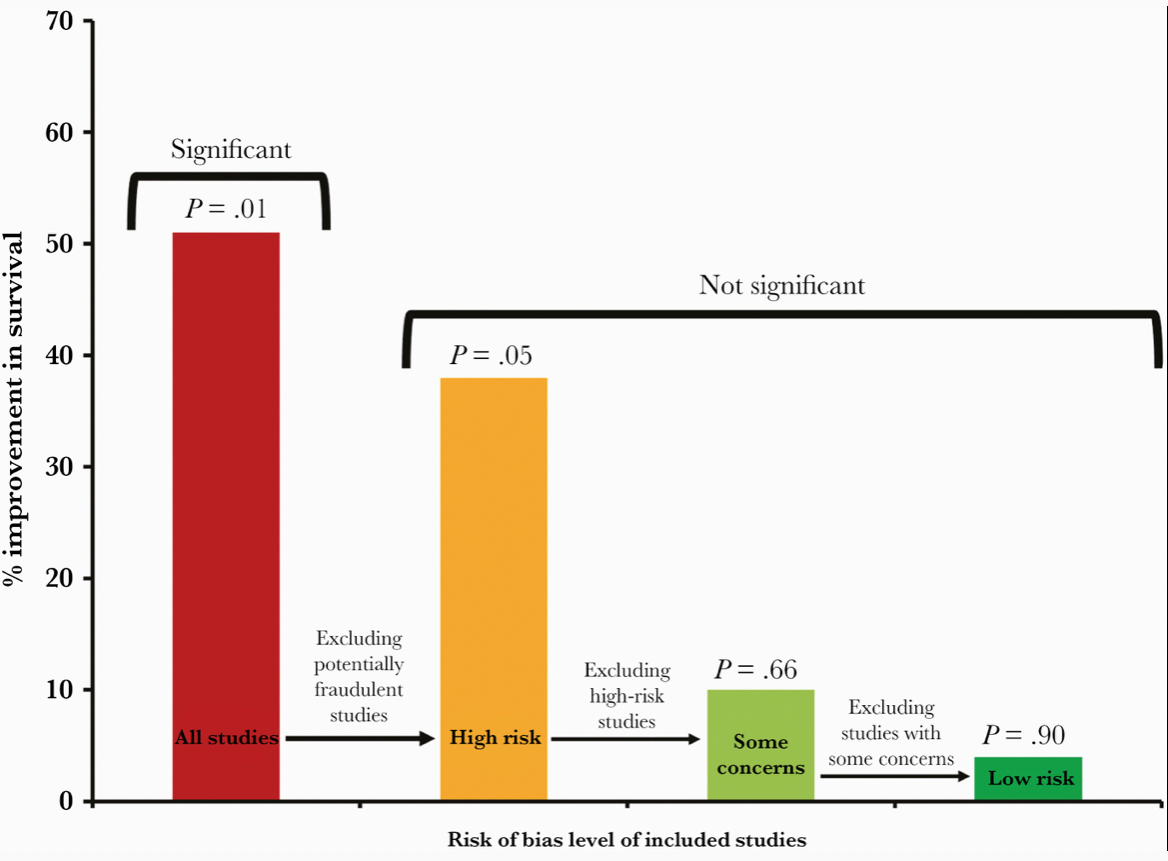
Read the full paper! But if you don’t have time, here’s the lesson:
These revised results highlight the need for rigorous quality assessments, for authors to share patient-level data, and for efforts to avoid publication bias for registered studies. These steps are vital to facilitate accurate conclusions on clinical treatments.
Indeed. And for whatever role I played as an editor in sustaining the confusion — and sometimes heated conflicts — over this potential treatment for COVID-19, I deeply regret it and apologize. I hope this post, and even more, Andrew’s revision, explain what happened, and hope we all can learn something from this mistake. Certainly I did.
Meanwhile, several large placebo-controlled trials continue to test ivermectin for COVID-19 treatment in outpatients. It still might do something (though at this point I’m not very hopeful). At least one of the studies — COVID-OUT — is fully enrolled, and ACTIV-6 is far along, so more data will be forthcoming soon.
Will these results be enough to quell the controversy about whether ivermectin has a role in treatment COVID-19, settling the issue once and for all? Let’s hope so.
February 4th, 2022
Prior COVID-19 Is No Guarantee of Immunity

19th century apothecary advertisement, Lowell MA. Product has fewer drug interactions than ritonavir-boosted nirmatrelvir.
I’m no immunologist — a fact made vividly obvious to me several years ago when asked to teach a weekly medical student section that included cases and problem sets. The challenge was that the course combined immunology and microbiology.
I was on much firmer ground with the microbiology than the immunology, the latter often a wonderfully complex and mysterious system.
So why, then, am I about to wade into perilous waters and write about something very much immunology-related, a subject I’ve already confessed to being an amateur at? Because to us ID specialists, the immune system plays a critical role in how our bodies respond, clear, and protect us from various infections. We may not be true experts, but it’s highly relevant to what we do.
Additionally, I’m watching a debate unfold among ID colleagues, public health officials, epidemiologists, the lay public, politicians, and yes, even advanced degree-holding immunologists. It’s a debate about a critical issue facing the globe as we march on to the third year of the pandemic.
Namely, does infection with SARS-CoV-2 confer protective immunity?
Views range from “yes, certainly” to “maybe, sometimes” to “it depends” to “absolutely not.”
Everyone can cite their favorite study to back up their opinions — whether it’s an epidemiologic analysis of reinfection rates, or the cases of reinfection after prior COVID-19 that are less (or more!) severe than the first, or an in vitro study demonstrating robust (but then waning!) antibody responses, or how cells from people with prior infection continue to mobilize protective cytokines, or contrarily how prior infection inhibits these helpful cellular responses.
Many of the people lining up on opposite sides of this debate are smart, have impressive credentials, and aren’t shy about expressing their opinions — hence sparks do fly.
This doesn’t help resolve the issue, because of course they cite mostly the scientific studies and opinion pieces that support their views.
So for the price of subscribing to this blog, here’s my non-immunologist’s take, and warning — it’s a messy one with no precise answer:
Prior infection confers some degree of protective immunity. It varies from person to person, depends on the severity of the disease, with “just right” being more protective than mild or severe illness. (Cue classic Goldilocks analogy.) It’s not durable, is not guaranteed, and certainly isn’t going to lead to herd immunity on its own anytime soon.
Nope, no herd immunity by April 2022, just like it didn’t happen by April 2021.
Incomplete immunity conferred by prior episodes of COVID-19 is one critical reason that studies of populations continue to show that the unvaccinated have a markedly higher rate of hospitalization and death than the vaccinated — even though prior infection is becoming more common.
Two years into the pandemic, with major population surges, this remains an undeniable fact everywhere it’s studied — even though a higher and higher proportion of the unvaccinated have already had the disease. If prior infection were strongly protective, the gap between vaccinated and unvaccinated in risk of hospitalization would decrease over time. It hasn’t.
Omicron only exacerbated this reinfection issue, with many of us seeing or hearing about patients with repeat infections, sometimes quite quickly after a first infection:
https://twitter.com/TheBlondeRN/status/1489608641858654219?s=20&t=bairQyDjErYe4NJziFpC9Q
Indeed, if we asked a group of primary care clinicians, emergency room folks, and other front-line providers to raise their hands if they had cared for or heard of people with more than one episode of COVID-19 — usually less severe, but sometimes more — 100% would have their hands up.
Incomplete protection from prior infection isn’t what any of us want to hear. But as we’ve learned again and again, wanting something from this virus doesn’t make it happen. The other night, someone in my family asked me whether now, as Omicron nabbed so many of us who previously escaped, can we at last move past this pandemic and get back to normal?
It’s such a good question!
I just wish I had a more reassuring answer.
In the meantime, let’s get everyone vaccinated, even those with prior infection. It’s a much more reliable way of getting protected, especially from severe disease.
January 28th, 2022
Required Learning Modules and How to Make People Learn

Mandatory learning modules were different in the 19th century.
Like thousands of others who work in my organization, I’ve just finished a stultifying annual task — several hours of required online learning modules that must be completed to keep my job.
How much do we look forward to them? Think annual car inspections, or tax returns, and multiply the boredom factor several-fold.
You might not be surprised to hear that the due date is … today.
You also might not be surprised to hear that as part of the process, I aggressively sought out ways to multitask while the videos ran in the background — cleaned my desk, filed papers, did low cognitive demand patient care tasks, repeated some arm strengthening exercises to prevent tennis elbow, checked the latest weather forecast (ugh).
Since the clever folks who make these modules programmed them to stop running unless the video or slideshow is the active window, could I have been the only one who opened the program on a second computer? Doubtful.
In short, these “learning modules” offer a prime example of how difficult it is to make people learn things when they’re in no mood to learn.
Without exception, everyone I know races to the finish, delighted to find their to-do list empty. Even this doc, despite his admirable quest for learning and growth. Find an elective in the catalog! You bet.
https://twitter.com/kkidia/status/1487036908039544833?s=20&t=IYIQhUl6HbTSF7V9-RHTzw
It’s not that the topics in these required modules are unimportant — anything but. Patient confidentiality, racism in the workplace, infection control, employee harassment, managing chemical spills and fires, what to do if there is an armed intruder in the hospital.
Sure we make fun of the fire extinguisher trivia required to pass the fire safety module (“The canister with a thin hose which contains water is a Type A fire extinguisher, used for ordinary combustibles”), or the incredibly obvious statements (“Chemical exposures can be dangerous”). Tornado safety measures will never be as relevant in New England as they are in Oklahoma.
But read that list from the above paragraph again. It’s important stuff!
No doubt organizations must, for their accreditation, demonstrate that their employees are dutifully notified about these issues, explain when and how to get help, and provide a list of resources where a person can go to get more information. Given the extraordinary range of activities and backgrounds in hospital workers, this cannot be an easy task. One size most certainly does not fit all.
So I’m sympathetic about the challenge.
I just think that from a pedagogical perspective, there has to be a better way — a less painful way — to learn the material, one that would allow the hospitals to check this box.
Maybe this owl can come up with something while keeping her egg warm. Lots of thinking time.
She can even use the time to finish her Healthstream modules.
January 18th, 2022
Novak Djokovic Thinks He Can Play by His Own Rules — Australia Says Think Again

YWCA Health Poster, 1920s.
There was a truly talented kid on my ninth grade basketball team — let’s call him Robbie G.
He was a terrific player, fast and confident on the court, always eager to play, and just brimming with enthusiasm for the game. Every time he scored, the team and crowd (small as it was) buzzed with excitement and shouted his name. A joy to watch.
But — and this might surprise you based on my introduction so far — Robbie was an immature jerk.
The rules didn’t apply to him. He did what he wanted during practice, and with few exceptions, the coach just shrugged and let him.
More from Robbie: He jokingly (ha ha) taunted the less-good players on the team, which meant just about everyone received some knocks. I certainly learned to avoid him both in the locker room and on the bench (where I spent most of my time during games).
When our team lost, or even worse — when he met his match in an opponent who guarded him closely — he became a petulant baby. These were not good times to cross his path, or heaven forbid try to comfort him.
This behavior carried over to non-basketball settings, and in school he was a terror. Sometimes a bully, sometimes only a disruptive nuisance, he quickly made his his presence known to everyone nearby — the effect amplified by a small group of less-talented (and equally annoying) sycophants.
I couldn’t help but think of Robbie while reading the news about Novak Djokovic, currently the best tennis player in the world. He can’t play in the Australian Open this year since he’s refused COVID-19 vaccination, and the Australian government will not approve his visa.
Like Robbie’s artistry on the basketball court, only scaled up to the adult and professional level, Djokovic’s tennis is a sight to behold. Here, let sports writer (and avid tennis fan) Joe Posnanski describe it (I’d never do it justice):
…He blends the most incomprehensible array of weapons — blazing speed, unmatched anticipation, a mathematician’s sense of angles, a tireless hunger to return every shot, a dream of a backhand and the best return of serve in tennis history — and turns every match into an art exhibit.
But … see what I wrote up there about Robbie, and the rules not applying to him? This fits the Djokovic affair perfectly. It also fits that Djokovic gave an interview and posed for a photoshoot for a media group after exposure to COVID-19 and testing positive by PCR.
I feel fine. Some of my tests were negative. See, the rules don’t apply to me.
High-level athletic ability facilitates this I-know-best view of the world. Here’s the recipe: A lifetime of winning. Adulation by friends, fans, teammates, coaches. Intense competitiveness. Suspicion of others. Wanting to beat others.
Mix these all together, and of course a person ingesting this potent brew is going to think they know best for themselves. Each win proves them right, reinforcing the problem.
In fact, we’re so used to hearing stories about rule-breaking behavior among professional athletes — how about football’s Aaron Roger’s definition of ‘immunized’? — that those who express thoughtful and generous sentiments stand out as model citizens. Tennis has two of these exemplars at ready hand, Roger Federer and Raphael Nadal. Federer, Nadal, and Djokovic — the “big three” — have dominated men’s tennis for over a decade.
Says Nadal, with searing accuracy, about the whole Djokovic affair:
If he wanted, he would definitely be playing here without a problem. Everybody is free to make their own decision – but there are some consequences, no?
Bravo. And let the record show that 97 out of the top 100 tennis players in the world are vaccinated. Djokovic, one of the three unvaccinated, refused the vaccine because he thought he could. His rules. The Australian government, citing their rules, said otherwise.
Back to Robbie G. for a moment. Remember, there’s another important difference between my basketball teammate Robbie and Djokovic, one that goes way beyond their relative fame, fortune, and chosen sport.
Robbie was 14 years old. Djokovic is 34.
January 12th, 2022
The Pandemic Life of an ID Doctor — in Graphic Form
Three works of art sit on my father’s desk in his office, gifts from his children from when we were in grade school.
From my brother Ben, there’s a little bear, or perhaps it’s some other burly quadruped — easily a B+ in his art class from the 1960s, likely an A- now with grade inflation.
A graceful ceramic bird represents my sister Anne’s contribution. It’s elegantly taking wing with the kind of naturalistic style one might have found from Bernini himself as a 6-year-old. I can just hear the teacher’s approval as it came out of the kiln — top-of-the-class kind of stuff.
My piece of “art” is a rock with three small shells stuck on awkwardly by Elmer’s glue. Ouch.
In short, in our family Anne got the bulk of the artistic skill (and I got the least — almost zero). Long-time readers may remember her cartoons from our ID caption contests, this figure of the Four States of Clinical Medicine (a particular favorite of mine, so clean), or this Fulyzaq, may it rest in peace.
I bring this up now because Anne kindly asked me what life is like for us ID doctors now 2 years into the pandemic.
I tried to explain it by saying that our usual distribution of work and non-work activities are constantly barraged by this big bully, COVID-19. If you picture your life as a pie chart, with each slice representing how you spend your time, along comes this scary, gigantic brute shoving everything else aside and making the rest of your life experiences and activities that much smaller.
Anne was kind enough to sketch it out for me — it looks something like this:
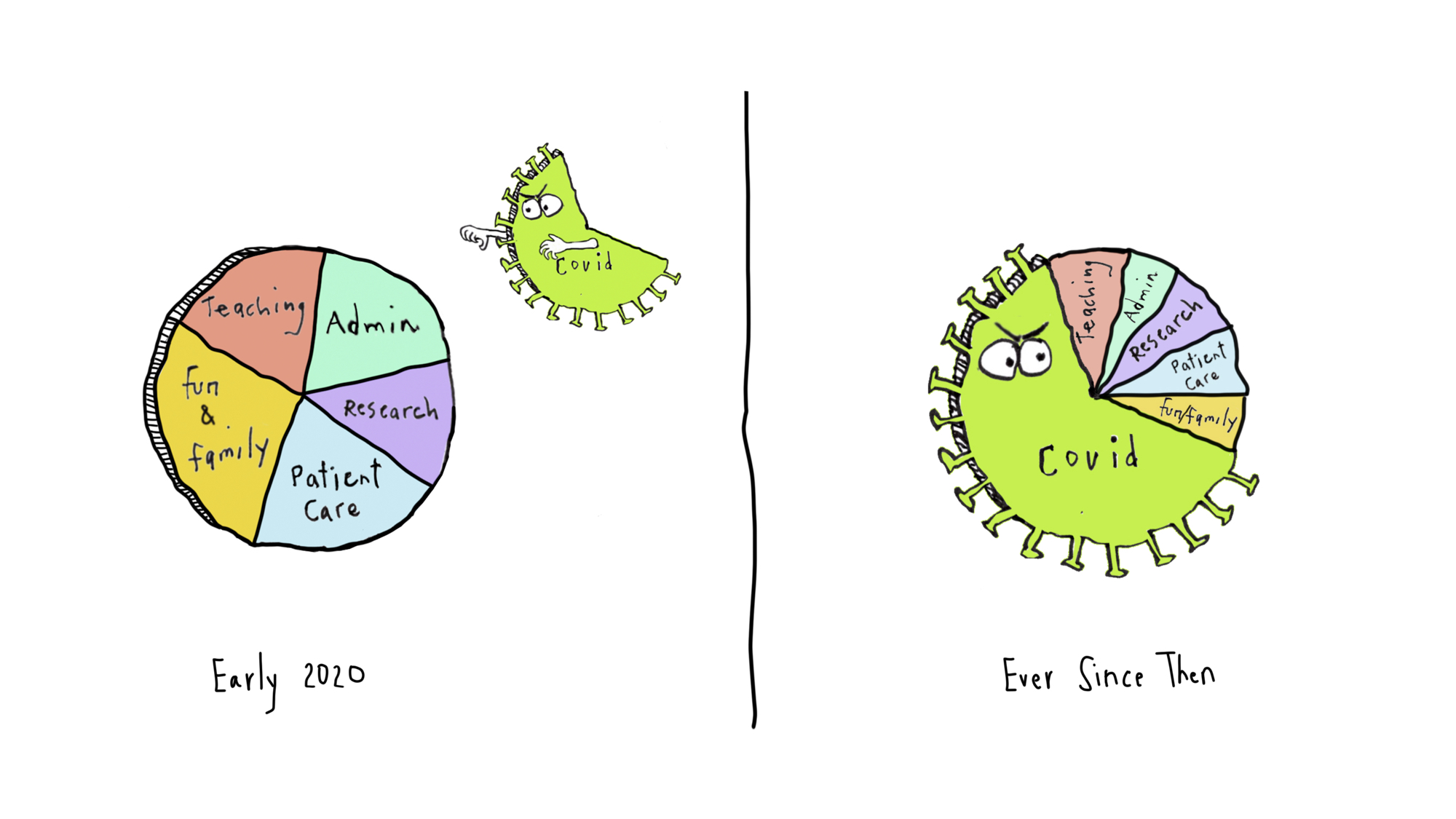
The compression of non-COVID stuff is a loss — it’s even more extreme during surges (now), with the COVID part occupying at least 80% of this pie chart. And 90% of our worries and disturbing dream content.
So what are the consequences for other work and life activities? Let’s consider a few, selected at random from my experience and that of my colleagues.
That friendly call to your patient who recently started HIV therapy to give them the good news about their first monitoring blood tests?
That research on molecular mechanisms of drug resistance in shigella?
That quality improvement project on optimal management of Staph aureus bacteremia?
That teaching module for medical students on the diagnostic approach to fever and rash?
And finally, that hike in the woods to the top of a nearby mountain with your family, fully disconnected from work?
All made that much smaller — or delayed or skipped — by this giant piece of pie insensitively hogging space in your life chart. There’s only so much time in a day.
Of course, this is understandable. We’re infectious diseases specialists, after all, and the pandemic is caused by an infection, a virus. And no doubt this is true for other clinicians in various specialties — emergency room doctors, primary care providers, pulmonologists.
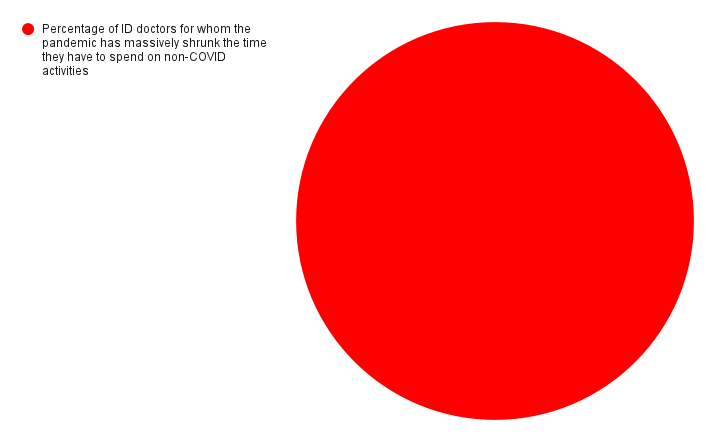
But I can guarantee what another pie chart looks like. It’s shows the percentage of ID doctors for whom the pandemic has massively shrunk the time they have to spend on non-COVID activities.
It’s a big circle, completely filled in, 100%. All of us.
Even I can draw that one.
December 30th, 2021
Omicron and Reduced Severity of COVID-19 — Some Good News We Desperately Need
We’ve been burned so many times making predictions about COVID-19 that I should post this conspicuously by my computer:

The famous pundit meant, of course, that “you don’t know anything” — we know his true intentions since he also said “Nobody goes there anymore. It’s too crowded.”
The “know nothing” quotation is a reminder that COVID-19 is a new disease. While we do in fact know more than nothing, we still have so much to learn. This applies to pretty much the whole COVID-19 universe, including its origins, virology, immunology, epidemiology, prevention, clinical manifestations, diagnosis, treatment, and complications. Constantly learning, constantly updating assumptions, guidelines, and messages.
With that humble start, I’m about to write a sentence that fills me both with excitement and, I confess, some trepidation.
It looks like Omicron causes less severe disease than prior variants.
Why trepidation? Well obviously I don’t want to jinx it, we’re still in the midst of a very major surge.
As anyone with a pulse knows, a lot of people have been diagnosed with COVID-19 in the past few weeks, more than we’ve seen at any point in the pandemic.
In fact, our world right now is a mess. (I’ve gone back and emphasized this sentence with bolding and italics, as some readers have told me they missed my acknowledging this stark truth.) Some have a mild or asymptomatic case, but some are miserably ill, recuperating at home. A smaller number are hospitalized with severe COVID-19, especially the unvaccinated or those with impaired immune systems. Some proportion of all these cases could end up with long COVID, a dreaded complication with still no clear treatment.
Plus, each new diagnosis — regardless of severity — enormously disrupts family, school, work, and social life. What I’m about to write does not in any way to diminish how awful the pandemic is right now for just about everyone.
But looking at the evidence and clinical experience on severity, I’m going to poke the beast and conclude that Omicron does appear milder.
First, the big picture — we have a record number of cases pretty much everywhere in North America and Europe.
(1) The massive number of infections in European countries is "real".
Yes, more people got tested for Christmas, and there have been some backlog issues in many countries.
But the 7-day positive rates have been rising rapidly, and nothing indicates that cases are "over-counted". pic.twitter.com/YFClWuXDYa— Edouard Mathieu (@redouad) December 29, 2021
However, despite this extraordinary spike in cases, deaths from COVID-19 are numerically lower than they have been at any point since way back in October 2020, more than a year ago. Record high case numbers and fewer deaths means that the global case fatality rate has dropped — it’s now below 1% for the very first time during the pandemic.
It’s even more encouraging when you consider that we are undoubtedly undercounting cases, both because people with mild symptoms are less likely to be tested, and because positive home tests are rarely reported. That denominator keeps getting bigger.
What these remarkable data tell us is that Omicron’s mutations may fool our antibodies, but our protection is far from useless — infection with Omicron occurs, but our primed cellular immunity (from prior infection or vaccines or both) quickly kicks in and takes it out. Go T-cells!
Second, let’s look at Omicron — the virus itself — in the lab, and compare it with other variants in its ability to inflict damage on various cells. Soon after Omicron appeared, a lab in Hong Kong reported that it grew less well in lung tissue than other variants.
Just one lab? Interesting, but let’s see what others find … and now we have no fewer than four others all reporting similar data, a veritable world tour that started in Hong Kong, but now includes Liverpool, Belgium, Japan, and Cambridge:
5 Studies, 5 Figures. All consistent, independent replications in vivo, in vitro. Omicron can't infect lungs or lung cells as well as prior variants.@KU_Leuven @SystemsVirology @GuptaR_lab @hkumed @LivUni pic.twitter.com/X9jbugx0vs
— Eric Topol (@EricTopol) December 29, 2021
Less virus activity in the lung could mean less lung damage, the primary organ targeted in severe — and fatal — COVID-19. Hope.
Third, let’s talk with the front-line clinicians — people like you and me. The doctors and nurses seeing cases in South Africa told us repeatedly in late November that this Omicron-related COVID-19 was different, that patients were less sick.
They generated data to confirm their impressions, yet somehow some northern hemisphere docs still didn’t buy it.
South Africa just had a devastating Delta surge, the thinking went, so there was natural immunity protecting them — the lower severity may not apply here. And remember, it’s summer down there. Plus, can the data be trusted if not collected here?
But now, our data and our anecdotal impressions match theirs — in plain speak, they were right. Anyone doing clinical care currently in the United States would agree that most cases we see right now are truly mild.
Not just mild in the over-inclusive category of “not requiring hospital care.” But mild like any generic endemic respiratory bug — remember those? Many of the clinical encounters with patients with COVID-19 occur over the telephone, and even their voice sounds different from prior variants — stronger, more confident they’ll recover, less afraid. That’s highly anecdotal, I know, but it’s just what I’m hearing.
So what does this giant Omicron surge, with a seemingly less-dangerous variant, mean for the future of COVID-19?
What does it mean for the “average” patient who gets it? How about for the immunocompromised, the elderly? For young children?
What does it mean for the logistics of patient care, work, schooling, getting together with friends and family, or just trying to live our lives in some tolerable way until the surge abates?
What should it mean from a policy or public health perspective?
What do these early data on cross-immunity with Delta mean? Sounds promising, but what does it mean for protection against future surges, or the inevitable next variant?
I could speculate, both on the optimistic and pessimistic side.
But I don’t know nothing.
Happy New Year, folks.
December 20th, 2021
Believe It or Not, We Already Have a Highly Effective Outpatient Antiviral Treatment for COVID-19

A pine tree. With a colorful birdhouse.
And that treatment is … (drum roll) … remdesivir.
Yes, you heard me right. Remdesivir, the very same antiviral with a checkered history in COVID-19 clinical trials, with some studies showing efficacy (sort of), others not much of anything.
What’s the truth here?
For that, let’s turn to what gets my vote for the most unheralded highly favorable clinical trial result since the start of the pandemic, the PINETREE study.
The trial randomized 562 outpatients with an increased risk for severe COVID-19 to daily intravenous remdesivir for 3 days or placebo. None had vaccination or treatment with monoclonal antibodies.
The results were unequivocally positive — remdesivir-treated patients had an 87% reduction in the risk of hospitalization or death, with no significant treatment-related toxicities. Key figure from the IDWeek 2021 late breaker presentation:
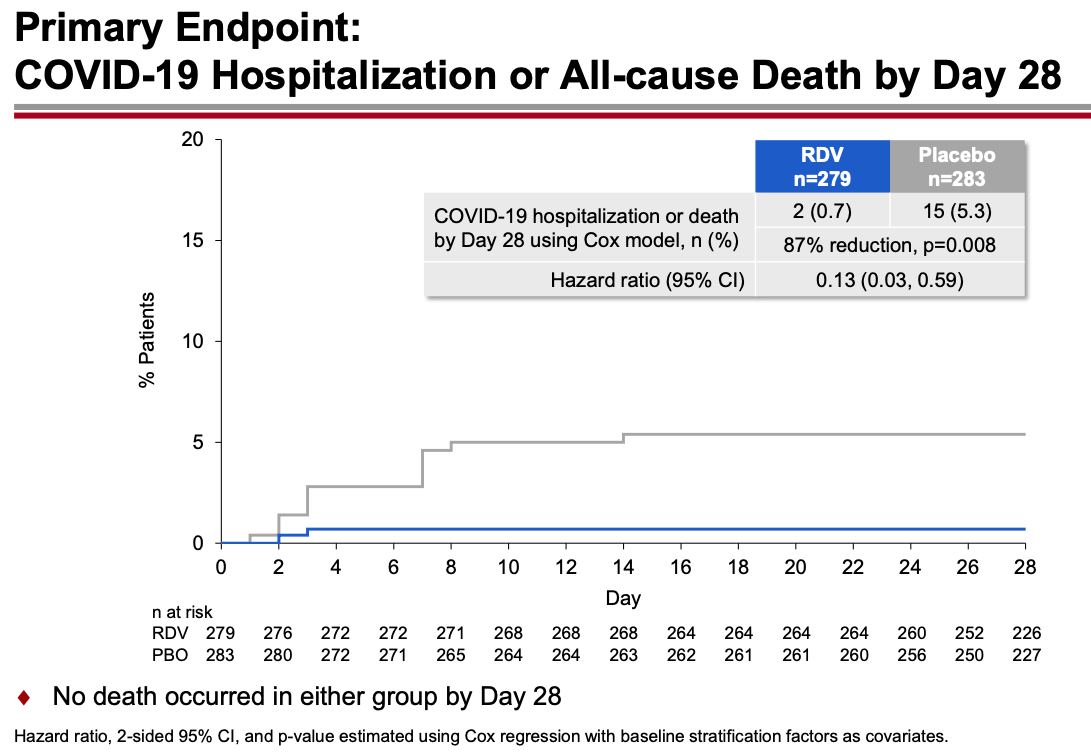
The contrast with the so-so results of the drug in hospitalized patients is striking, and underscores the importance of early antiviral treatment for this disease. By the time the immune-mediated organ damage kicks in — which is when most hospitalizations take place — it’s often too late for antivirals to have any effect.
Note that remdesivir given early in the course of illness to outpatients yields comparable results to the monoclonal antibodies and nirmatrelvir plus ritonavir (Paxlovid), and better results than molnupiravir. (Yes, caveats about cross-study comparisons understood.) Furthermore, remdesivir has been available for months, and is the only fully FDA-approved treatment for COVID-19 — which means clinicians can prescribe it in whatever setting they deem useful.
But it’s hard — OK, nearly impossible — to find anyone who has actually used remdesivir in a nonhospitalized patient:
The PINETREE study showed that remdesivir daily for 3 days in nonhospitalized, high-risk individual with Covid19 reduced the risk of hospitalization by 87%.
Have you or anyone you know used it this way outside of a research study? https://t.co/o9e0IBPH0F— Paul Sax (@PaulSaxMD) December 19, 2021
The reasons so few have adopted this treatment are obvious. Setting up outpatient intravenous therapy urgently is a challenge even under the best of circumstances — and doing so for patients who are acutely ill and have a highly contagious infectious disease only makes it more challenging.
The study sponsors recognized these hurdles and stopped the study early because of the availability of single-infusion monoclonal treatment and vaccine availability, both of which seemed destined to make the study results of limited clinical utility.
But with Omicron’s arrival, the results of PINETREE instantly become quite relevant. Omicron is so highly contagious and vaccine evasive that many people who have been “fully vaccinated” (time to retire that phrase, by the way) will contract COVID-19. My (very funny) British ID colleague Neil Stone describes it as follows:
Forget airborne, this thing seems to spread by telepathy — feels like you can catch it just by THINKING about it.
Furthermore, the most widely used monoclonal antibodies in current use — casirivimab and imdevimab and bamlanivimab and etesevimab — don’t neutralize Omicron. Sotrovimab, which is predicted to retain some activity, is in very short supply. The oral antivirals nirmatrelvir and molnupiravir are not yet available, and even once they get approved, supplies will be severely limited. Note again that molnupiravir reported lower efficacy than remdesivir, and furthermore has mutagenesis concerns.
My late colleague Francisco Marty — a brilliant clinical researcher — proposed studying remdesivir as a single IV dose, analogous to the one-dose baloxavir and peramivir treatments for influenza. This would greatly facilitate outpatient and emergency room treatment. Remdesivir infusions don’t take long, and require no post-treatment observation. Unfortunately, the study never went forward.
Maybe it’s time to revive the idea? Start a clinical trial now comparing 1 vs. 3 doses, using a noninferiority design?
Regardless, we’re going see a lot of COVID-19 in the next few weeks. Since most PCR assays do not report “spike gene target failure” — a hallmark of Omicron — clinicians will not know in real time whether these cases are Omicron or Delta, and hence whether the currently available monoclonal antibodies will work.
But based on other regions where Omicron appeared, the dominance of this variant is all but inevitable and will occur rapidly. This experience in Houston is typical, one we’re seeing in multiple areas in the United States right now:
Sunday Update: The Omicron variant is now in Houston in full force, accounting for 82% of new symptomatic Houston Methodist #COVID-19 cases as of earlier this week.
#Omicron became the cause of the supermajority of new Houston Methodist cases in less than three weeks. pic.twitter.com/XL5C31P4As— S. Wesley Long (@drswlong) December 19, 2021
Why not prepare for this change by shifting the resources we’ve developed for intravenous monoclonal antibodies to intravenous remdesivir? Let’s offer this first to our highest risk patients — people who are B-cell depleted, transplant recipients, other severely immunocompromised.
It won’t be easy, but it will be worth it. Let’s use all the effective tools we have to treat this disease, and that includes remdesivir for high-risk outpatients.
December 15th, 2021
Do We Need to Remind People that COVID-19 Is Truly Awful?
Last month, I read a deeply harrowing description of what it’s like to be hospitalized with severe COVID-19. I strongly urge you to read the full thread:
https://twitter.com/CRStoli/status/1458600993621430272?s=20&t=km6XxJ5IDsQGQKsygjXfWg
Something about the plain, direct language he uses to describe the patient experience hits home here like few other depictions I’ve read — it rings true in so many ways.
And many of us in healthcare might forget just how dehumanizing and uncomfortable hospitalization can be — not just the severe discomfort of being critically ill, but the added insults of daily early AM blood draws, terrible food, scratchy pillows, and loss of privacy.

Peter in the captain’s chair.
The author is Chris Stolarski, associate director of university communication at Marquette University in Milwaukee, who was kind enough to talk with me further on an OFID podcast, which is also embedded at the bottom of this post. You can tell from our chat that Stoli (that’s what everyone calls him) is by nature an upbeat person — a Groucho Marx fan and music lover, with a particular fondness for old dachshunds. Here’s his pup Peter, by the way.
Still, Stoli is quite clear that he nearly died — his hospitalization was a terrifying nightmare, and his recovery far from easy, the illness leaving him with permanent nerve damage and tachycardia. He’s hopeful that telling his story will convince more people to take COVID-19 seriously and get vaccinated. (He contracted COVID-19 before vaccines were available.)
Why is this still important? Well just this week, The Atlantic published a piece entitled, Where I Live, No One Cares About COVID, by Matthew Walther.
In a bemused, and at times openly mocking tone, he cites the ongoing concerns about COVID-19 as the domain of the coastal, urban elites. For him and his family, living in rural Michigan, life has been back to pre-pandemic normal for nearly two years.
Here’s a typical passage, in which he openly ridicules media coverage of how to gather safely during the holiday season:
As Christmas approaches, we can look forward to more of this sort of thing, with the meta-ethical speculation advanced to an impossibly baroque stage of development. Is it okay for our 2-year-old son to hug Grandma at a Christmas party if she received her booster only a few days ago? Should the toddler wear a mask except when he is slopping mashed potatoes all over his booster seat? Our oldest finally attended her first (masked) sleepover with other fully vaccinated 10-year-olds, but one of them had a sibling test positive at day care. Should she stay home or wear a face shield? What about Omicron?
I don’t know how to put this in a way that will not make me sound flippant: No one cares.
Yes, he’s an excellent writer. Also yes, he’s deeply wrong about this last sentence — No one cares should more accurately really read, I don’t care. Otherwise, he’s guilty of the same echo-chamber bias he’s leveling at “the professional and managerial classes in a handful of major metropolitan areas.”
Readers of this site will no doubt be aware that Walther chose to write this piece as cases and hospitalizations due to COVID-19 continue to increase rapidly in most of the United States — including in Walther’s state of Michigan:
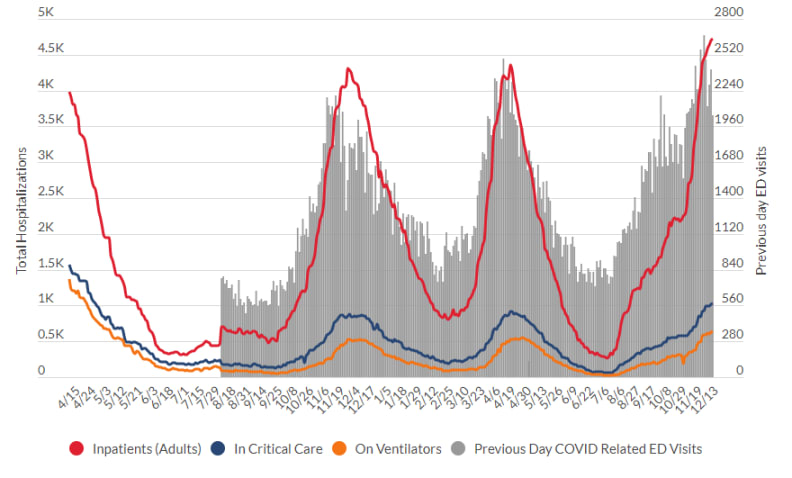
Source: Michigan Department of Health and Human Services
We’re sadly on the same grim trajectory we were last winter — and this is before the highly transmissible Omicron variant makes a major push into our country. We might deeply want COVID-19 to be over, but this doesn’t magically make it happen — the virus couldn’t care less.
Not surprisingly, Walther’s piece triggered the ire of many (raises hand!), in particular front-line healthcare workers who can’t hide from the disease. Here’s a particularly good (and angry) commentary from University of Colorado’s Dr. Josh Barocas, who kindly substituted * for certain letters so I could quote it here on these pages:
As an ID physician, frontline worker, & public health professional, I think I’ve generally held my sh*t together pretty well over the past two years (not lashing out, being civil) but this garbage @TheAtlantic article undid me (let me explain)-please readhttps://t.co/oWyLwtKmcj
— Josh Barocas, MD (@jabarocas) December 14, 2021
Well said, Josh. Because the answer to the question posed by title of this post is clearly YES.
(Transcript here.)
December 13th, 2021
ID Learning Units from Inpatient ID Consults
Nerve cells in a dog’s olfactory bulb, from Camillo Golgi’s Sulla fina anatomia degli organi centrali del sistema nervoso (1885).
Need a break from all-things COVID-19? Feeling OH-verwhelmed by OH-muh-kron?
(I guess that’s how some pronounce it … or AWE-mee-kron… or oh-MIKE-ron … or who knows. Two things for sure — there’s no “n” after the “m”, and it’s a drag regardless of how it’s pronounced.)
To cheer everyone up, here’s a palate cleanser of non-COVID-19 ID Learning Units from a stretch on the inpatient ID service earlier in the fall. I’ve been storing them up just for today.
Here are the criteria for inclusion:
- Has to be related to a case.
- Has to be posted each consecutive day. (Careful readers will note I missed one so did two on one day. Mea culpa.)
- Has to have a reference.
- Has to be interesting.
- Has to have no patient confidentiality issues.
And for this batch, it has to be a non-COVID-19 topic. Though some may find it hard to believe, there are other infectious diseases out there — in fact the entire field of ID existed before SARS-CoV-2 ever jumped its way to humans and started this dismal pandemic.
As always when on service, I’m very grateful to have worked with a couple of highly energetic and smart ID fellows, an ID pharmacy team (faculty and resident) who taught me something daily, plus a medical resident who seems destined (I hope) to be a great future ID doc.
Hooray for that!
Off we go, each with a bit of commentary about why they were selected:
#1:
Day #1: NADIA trial — after rx failure, DTG non-inferior to DRV/r when given with either TDF/3TC or ZDV/3TC, despite *extensive* NRTI resistance. Huge implications for management of both viremic and suppressed patients. https://t.co/Mjx6EWoH4J
— Paul Sax (@PaulSaxMD) October 13, 2021
NADIA is one of the most practice-changing studies in HIV medicine in a while — which ironically will be more relevant in clinical practice for those already suppressed than those with treatment failure, even though the latter is the study population.
Key question — can essentially all people with HIV on 3-class regimens or NRTIs plus boosted PIs due to resistance switch to tenofovir/FTC plus DTG? Or even simpler, to BIC/FTC/TAF? I think so, provided they have full susceptibility to INSTIs.
#2:
Day #2: Xpert MTB/RIF outperforms AFB smear in ruling out TB in low prevalence settings, with two negatives having a 100% neg predictive value. An important advance in hospital infection control. @annieluet https://t.co/zR5cxxTMQk pic.twitter.com/NEUY2I4XLN
— Paul Sax (@PaulSaxMD) October 14, 2021
The results of this study greatly expedite the tuberculosis rule-out, which in low-prevalence settings is done on many patients with an extremely low pre-test probability of TB. As always, no test is 100% perfect in the real-world setting, which means that for high pre-test probability cases, we might need to keep up precautions even with negative testing.
#3:
Day #3: For pts with recurrent cellulitis, this RCT showed that penicillin reduced the incidence of cellulitis from 37% to 22% over a median of 25 months of follow-up. NNT to prevent 1 episode of 5. Practice-changing study for many clinicians. https://t.co/b27R12pP3D
— Paul Sax (@PaulSaxMD) October 15, 2021
I find myself citing this study repeatedly, probably because there are so few clinical trials of long-term antibiotic use that so clearly demonstrate benefit. And don’t forget the compression stockings, another effective measure!
#4:
Day #4: Small comparative clinical trial found T/S as effective as sulfa-pyr for toxo encephalitis, and better tolerated. Results reinforced by observational data and clinical experience globally. Often we switch to T/S at d/c — what's your practice? https://t.co/6mI3yiCZxg pic.twitter.com/KObKFzabTJ
— Paul Sax (@PaulSaxMD) October 16, 2021
This is the only randomized trial comparing TMP/SMX to pyrimethamine plus sulfadiazine for HIV-related cerebral toxoplasmosis, and it’s woefully underpowered (a fancy way of saying, “too small”). But globally many more use TMP/SMX for this infection — it’s more readily available, less expensive, and much easer for patients to take.
Since it’s highly doubtful that there ever will be another controlled clinical trial, at what point do guidelines shift to recommend TMP/SMX over pyrimethamine-sulfa solely on the weight of clinical experience? I’d vote soon, since it’s not as if the pyrimethamine-sulfa strategy itself was ever subject to a controlled studies. (And no, I don’t know why this 1998 study was reprinted in December 2020.)
#5:
Day #5: The Staph intermedius group includes several coag pos staph spp that are the predominant cause of soft tissue infection in dogs — but can rarely also cause invasive disease in humans. Woof https://t.co/qCvwTlZcdI @NeilanAnne pic.twitter.com/wZ6aPGPKpY
— Paul Sax (@PaulSaxMD) October 17, 2021
Hi Louie! A self-indulgent post about a little known dog-related fact.
#6:
Day #6: Cefepime neurotoxicity is most common in older patients with renal dysfunction (heard that one before?), and should be considered especially in those with myoclonus. Don't forget dose adjustment in renal disease! https://t.co/Rl9Wcm6Gwd pic.twitter.com/JEbqlOBGqz
— Paul Sax (@PaulSaxMD) October 18, 2021
Anecdotal impression: Among hospital-based clinicians (hospitalists and medical residents), there is much greater awareness of this notable adverse effect of cefepime — especially with the decline in use of vancomycin plus piperacillin-tazobactam due to concerns about its renal toxicity.
#7:
Day #7: S maltophilia is intrinsically resistant to many agents (n.b. carbapenems), but often retains susceptibility to T/S, levo, ceftaz, mino. T/S usually listed as Rx of choice, but no comparative studies. Meta-analysis: FQ at least as good as T/S. https://t.co/4Rd48eNzDq pic.twitter.com/HH29KqOX0a
— Paul Sax (@PaulSaxMD) October 19, 2021
“Steno” is such a tough bug to treat, and TMP/SMX at high dose can’t be used for everyone. Spoiler alert — another good observational study on TMP/SMX vs levofloxacin about to be published soon in a journal near you.
#8:
Day #8: Cefiderocol enters bacterial cells using active iron transporters & is active against many MDR GNR. C/w best available Rx, cefiderocol similar to best therapy vs CRE, but w/ numerically more deaths — cause unclear. Chance due to small #'s? Other?https://t.co/s7pOmv0rb3 pic.twitter.com/kpenANJZsR
— Paul Sax (@PaulSaxMD) October 20, 2021
This is one of those inexplicable clinical trials, with an important outcome — deaths! — happening more in the experimental cefiderocol than the control arm, for reasons that still are not clear. (Reminds me of the early clinical trials of bedaquiline.) I suspect it’s because the heterogeneity of the patients with these infections is so huge that there’s the chance of death for multiple noninfectious reasons too, but I don’t know. For now, as we await more data, cefiderocol is increasingly used for treatment of highly resistant gram-negative infections in the hospital.
#9:
Day #9: How quickly can antibiotics turn blood cultures negative? Fast! The rate of pos blood cultures reduced by >50% just an hour after IV antibiotic Rx — but many will still be positive, so getting cultures in septic pts still useful. https://t.co/XOywNPd5Ob pic.twitter.com/xOP4mmI960
— Paul Sax (@PaulSaxMD) October 21, 2021
Reminder to emergency room, primary care, and urgent care clinicians everywhere — get blood cultures before starting antibiotics. Thank you very much, you’ve just made a lot of ID doctors very happy.
#10:
Day #10: Can we use cefazolin for treatment of central nervous system infections due to MSSA? Good concise summary of the data, pro and con, here. h/t @BWDionne https://t.co/VskwYvLi3Z pic.twitter.com/RJikvkJFrr
— Paul Sax (@PaulSaxMD) October 22, 2021
This particular question — can we use cefazolin over oxacillin or nafcillin for CNS infections due to MSSA — comes up all the time. The short answer is probably yes, with the bad reputation of cefazolin for this indication likely a carry-over from observations about another first-generation cephalosporin, cephalothin.
#11:
Day #11: In RCT of linezolid vs vanco for MRSA pneumonia, clinical response (PP analysis) was significantly higher with linezolid. Similar results in the ZEPHyR study (2013). Both had methodological issues, but data suggest it's as least as good. https://t.co/LXWc5m20op pic.twitter.com/2DNUkC6Gc8
— Paul Sax (@PaulSaxMD) October 23, 2021
Back when linezolid was a branded antimicrobial and cost a ton, many didn’t believe this company-sponsored clinical trial, discounting the results on various methodologic issues.
However, a second study found the same result (linezolid better than vancomycin), and hence this is one recommendation I frequently make when consulted on a case of MRSA pneumonia — switch the vancomycin to linezolid.
#12:
Day #12: Chemoprophylaxis is recommended for close contacts of patients with meningococcal infection. How about exposure to culture-positive respiratory secretions, e.g. from pneumonia or pharyngitis? CDC says no — interesting distinction. https://t.co/yrVwU6NSTZ pic.twitter.com/vpBAte42Dy
— Paul Sax (@PaulSaxMD) October 25, 2021
Since I don’t really understand this difference in recommendations for preventive therapy, allow me to quote Dr. Patrick Hickey: “Meningococcus is one of those infections for which every time there is a possible exposure physicians dutifully review the guidelines and then (more often than not) don’t follow them.” Agree!
#13:
Day #13: Impaired renal function is a risk factor linezolid-related thrombocytopenia. According to @BWDionne, we may one day see recommendations for reduced dosing (600 mg daily?) in patients with low eGFR. https://t.co/gYYygjmvXi pic.twitter.com/OQD0jNZo3p
— Paul Sax (@PaulSaxMD) October 26, 2021
More on linezolid! A reminder that impaired renal function is a risk factor for drug toxicity, which can be severe. Seems like dose reduction for this drug in some patients would be warranted, but is not now currently recommended.
#14:
Day #14: After suppression with boosted PI (bPI) plus NRTI as 2nd-line ART, maintenance Rx with bPI plus 3TC has high rates of success, even with M184V — better than bPI monoRx. One of many studies showing ongoing antiviral effect of 3TC even with M184V. https://t.co/kEVOaHLMHK pic.twitter.com/Cq30jbuBQq
— Paul Sax (@PaulSaxMD) October 26, 2021
Monotherapy studies with boosted PIs and dolutegravir have invariably failed despite their high resistance barrier, with more virologic rebound and development of resistance. As demonstrated in the NADIA trial (see #1, above), this can be overcome with just partial NRTI activity — which here in MOBIDIP is lamivudine despite the M184V mutation.
That’s a wrap! I’ll be on again over the holidays, will likely do the same.
Now enjoy this amazing visual (just a clip) of an extraordinarily beautiful piece — Un Sospiro (A Sigh), by Franz Liszt, played and displayed in a novel way. In dark times, it’s helpful to remember that human beings can do remarkable things.
https://twitter.com/YouTube/status/1350533338998693891?s=20
Plenty more where that come from!