An ongoing dialogue on HIV/AIDS, infectious diseases,
August 12th, 2021
Could This Be Our First Effective, Inexpensive, Widely Available Outpatient Treatment for COVID-19?

The Geometric Landscapes of Lorenz Stoer (1567)
It’s fluvoxamine.
This rarely used antidepressant, long off-patent, has quietly been going through high-quality clinical studies for treatment of COVID-19. It certainly won’t be endorsed or promoted by any deep-pocketed pharmaceutical company, but deserves some attention nonetheless.
Here’s why I think we might finally be onto something with this “repurposed” drug, even after stumbling numerous times with hydroxychloroquine, lopinavir-ritonavir, ivermectin, azithromycin, doxycycline, colchicine, et al.
First, there is a legitimate mechanism of action — actually, multiple mechanisms, as it has anti-inflammatory, anti-platelet, and potentially antiviral activity independent of its psychoactive properties. If you want to get into the weeds, read this nice summary. But of course many drugs have in vitro mechanisms of action that don’t pan out.
Next, Dr. Eric Lenze and colleagues published a small double-blind clinical trial — well-designed and conducted — which showed benefit. Out of 152 participants enrolled, clinical deterioration occurred in 0 patients treated with fluvoxamine vs. 6 (8.3%) patients treated with placebo, a difference that was statistically significant.
But the problem with such small studies is that a tiny shift in outcomes for the treatment group would substantially change the conclusion. In other words, the results were “fragile.” The authors appropriately concluded that further larger studies were necessary.
After this trial, there was an observational study of opt-in versus opt-out fluvoxamine among newly infected workers at a horse racing track. Despite having more symptoms at baseline, the opt-in group receiving fluvoxamine had better outcomes than those who declined treatment — specifically, 0/65 hospitalizations for fluvoxamine, vs. 6/48 who chose observation only.
But the observational nature of this study also couldn’t provide a high enough level of evidence to change practice. What if the people choosing fluvoxamine were just more “health seeking” — and hence healthier — than those who declined, biasing the result? A highly plausible explanation for the results.
Still, these studies got enough attention to warrant further research, and even a spot on 60 Minutes. The research includes the innovative TOGETHER trial, led by a multinational group of investigators primarily in Canada and Brazil.
Here’s the “adaptive” study design, which shows the tested candidate drugs and the novel way the investigators drop unsuccessful treatments:
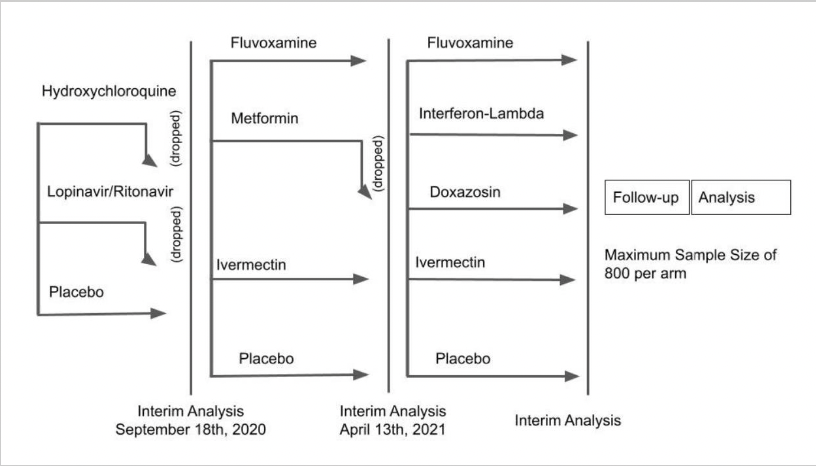
Eligible participants must have had symptom onset within the previous 7 days, a positive test for SARS-CoV-2, be older than 18, and have at least one risk factor for disease progression. The primary endpoint for these outpatient treatments was a composite of emergency room visits or hospitalizations due to the progression of COVID-19. The participants enrolled in 10 study sites in Brazil.
The group already published the negative results of their first study, showing that neither lopinavir-ritonavir nor hydroxychloroquine prevented progression to hospitalization or death better than placebo — which means those treatments have been appropriately dropped.
Time to move on to the next bracket, which included fluvoxamine, metformin, and ivermectin! Interim results were presented for the first time last week at an NIH meeting. The metformin didn’t do much of anything, and has been dropped; the ivermectin did a bit more, but still nothing practice-changing or statistically significant, with a relative risk of progression of 0.91 (95% confidence interval 0.69-1.19).
But the fluvoxamine treatment was much more promising. Among the 1480 participants randomized, fluvoxamine reduced the risk of disease progression by 29% (thanks to the lead investigator Dr. Edward Mills for this updated slide):
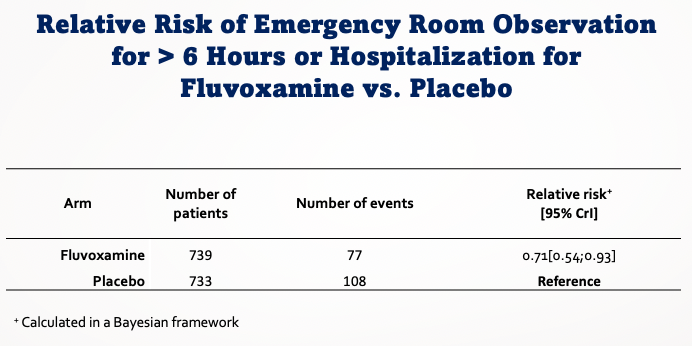
Most of the secondary endpoints also favored fluvoxamine, though the differences were not always statistically significant given the smaller event rate. No data on safety were presented, but Dr. Mills verbally stated that there were no unexpected toxicities.
No, an interim analysis of an ongoing study is not enough to change treatment guidelines — but it’s getting close, especially given the lack of other options and the favorable safety profile. The results are strong enough for the investigators to stop this comparison in this study, and no doubt a pre-print and submitted paper should be coming soon for further review.
Look, we’ve all been burned by promising studies of these repurposed drugs, and it’s quite reasonable to reserve final judgment until we see the complete data, and even other studies. Both the University of Minnesota COVID-OUT study and the NIH’s ACTIV-6 study include fluvoxamine arms.
But this already feels different from hydroxychloroquine and company given the high quality of the research. And it raises many interesting questions, including:
- Would the results be additive to monoclonal antibodies, which we know work well in early disease but remain limited in availability, expensive, and cumbersome to administer?
- How about combined with inhaled budesonide? Or with molnupiravir? (As an ID specialist with a research focus on HIV, you can tell I think combination therapy is a very good thing.)
- Should it be tried in inpatients, especially for those requiring oxygen, for whom anti-inflammatory approaches seem most beneficial?
- Would it work in other countries?
- On a global level, would fluoxetine be just as effective, as this SSRI is far more widely available?
- What should clinicians do now? Should they prescribe fluvoxamine for newly diagnosed patients with COVID-19? If so, for which ones?
No, I don’t have the answers. But this looks like progress, which during a pandemic is always great to see.


Another informative, clarifying blog post. Thanks!
Maybe it’s just my nature but I’m skeptical. Many of the purported mechanisms of action appear to be common to the SSRI class in general and given millions of people are taking these medications I would think a real benefit would have been detected. 60 minutes is doing no service by giving people hope before high quality trials are actually done. Let’s focus on getting people vaccinated.
But some high-quality trials HAVE been done, and we need BOTH vaccinations AND early treatments. Appropriate focus on vaccination should not mean ignoring the need for early treatments that can be given to outpatients with COVID-19 to potentially prevent hospitalizations and deaths. As far as a real benefit of SSRIs in general being detected, an apparent benefit of SSRIs has been detected in some observational studies by Nicolas Hoertel and colleagues. See Hoertel et al. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. Mol Psychiatry 2021 Feb 4. doi: 10.1038/s41380-021-01021-4.
Thanks for the great update! But a composite endpoint? What if the events in the fluvoxamine group were mostly hospitalizations and those in the placebo mostly “just” ER visits? I imagine the researchers looked at this, but I sure would want more information in this direction…
As you say, fluvoxamine isn’t widely used – but wouldn’t it be relatively straightforward to do a case control study of COVID patients on/not on SSRIs? For example, by the VA or other large health care organization? That would give an idea whether it would be worth working on any of the other SSRIs.
(I’m not volunteering to do it, by the way – no funding!)
Or is it already done/being done and I missed it?
I just hope the next “VA” study involving Covid is better than the one they did on HQC. Hopefully, if your volunteerism is accepted, you’d understand that giving a drug to the worst of the worst and people die does not mean the drug is the culprit. And hopefully you know how to link the data to the conclusions. It was an embarrassment to the medical/epidemiological education system in this country.
Thanks for this very informative update, Paul. Just a minor quibble: fluvoxamine isn’t used rarely. It’s used quite a bit for people with obsessive-compulsive disorder. Is it the first SSRI we turn to for depression? No, not usually. But it can be efficacious in OCD, as can several other SSRIs. I have several patients with OCD doing well on fluvoxamine. I sure hope there won’t be a shortage of it in the future, if it becomes more widely used for COVID!
Thanks for this correction, Loretta! It’s true that I should have written “rarely used for depression”.
-Paul
Recently a pharma company mentioned good response to fluvoxamine and itraconazole (I don’t recall if they were as standalone medicines or in combination). Itraconazole has been reported to be useful for basal cell ca and some visceral cancers, as add-on therapy. Prof. Dr. Sudhir Pujara, MD, India.
A thousand thanks for encouraging an effective and inexpensive medicine for this pandemic. By the same token may I propose a method to increase the efficacy and availability of all anti-COVID vaccines. Why not given them by intradermal instead of intramuscular injections. It is not only inexpensive, not only free of charge, but even money-saving. Intradermal vaccination can be given at a small fraction of the intramuscular dose (down to 1/10) with improved efficacy due to the high concentration of antigen presenting cells and other immune-active cells. This has been proven in many other vaccines. So, without further spending we can expand the supply of any vaccine up to ten times at no extra cost apart from the syringes and needles. At that dose we can expect, and convince vaccine-skeptics, that the vaccine will be less toxic and more acceptable. With luck it might even be more protective by recruiting more dendritic cells in the skin to expedite the immune process.
bethrees@netspace.net.au
IM route is used because the mRNA vaccines rely on the muscle cells to produce the spike protein, where it presents on the surface of the cell. This way the distribution of the spike protein is ostensibly limited, (and less likely to latch onto ACE2 receptors on any other organs or tissues), while still triggering the immune response.
IM route is used because the mRNA vaccines rely on the muscle cells to produce the spike protein, where it presents on the surface of the cell. This way the distribution of the spike protein is ostensibly limited, (and less likely to latch onto ACE2 receptors on any other organs or tissues), while still triggering the immune response.
careful assuming all ssri’s work the same way, they don’t necessarily have a COVID 19 effect through serotonin regulation.
this particular ssri is an agonist for the sigma-1 receptor, through which it controls inflammation (https://pubmed.ncbi.nlm.nih.gov/33959018/). a mechanism that has implications in platelet aggregation, mast cell degranulation etc. SSRI’s of other sorts sometimes hit this button.
using the big VA data base is a good idea. any VINCI wizards reading this?
NGK
The lower effect size for fluvoxamine in this trial was primarily due to the average 4 days from symptoms to start of treatment. The P1 variant requires immediate treatment.
It seems physicians have forgotten the precautionary principle of medicine.
2 people have died from fluvoxamine overdose in the last 40 years. Given for 14 days, it’s doesn’t significantly affect one’s mental state.
So you have a safe drug which is now shown effective in 3 studies.
If you just got COVID, would YOU wait until the paper came out so you could look at the data? I seriously doubt it.
A real doctor would do what David Seftel did at the racetrack: save lives. Not a single person in that trial regrets taking the drug. It made such a huge difference that even the track management noticed and wanted prescriptions in case they got COVID.
The protection is obvious to anyone who observes the two groups. This is why the track employees went from a 30% take rate in Seftel’s study to 100% take rate.
David Seftel’s had 77 patients, none of whom were hospitalized and none had long haul COVID. If the drug didn’t work, how do you explain that?
As Paul mentioned, this is off patent, and is very inexpensive. Their presentation shows the exclusions, and I noticed current use of an SSRI is not on the list. That’s good news, since so many people take SSRIs. I hope that later reports will mention how many patients were already on an SSRI, and how they managed this.
Addendum to my post – later in the presentation, he mentioned that current use of another SSRI was “not a concern” because of the lack of use of SSRIs in this low-income population. There will need to be a plan, particularly with SSRIs with a long half-life (fluoxetine).
Good
Brilliant summary of THE leading priority for #COVID-19 – safe/effective/cheap early intervention – Thnxs Paul
What kind of doses were used for fluvoxamine in COVID patients?
I too would like to know the dose used. I have a lot of patients on SSRI’s in my Women’s Health Practice- It would be interesting to see if they had milder covid symptoms.
Steve Kirsch, you are right. The concept of doing double blind placebo trials during a pandemic is not only illogical, it’s also unethical. Who would want to participate in something like this where there’s a 50/50 chance you get the placebo? No one in their right mind ! We should have listened to Professor Raoult. If others would have jumped in and helped him develop a treatment, instead of attacking his methods, this pandemic would have been over long ago. These archaic processes, that we are determined to live by, and defend at all costs, have led to needless suffering and death. It’s never too late to do the right thing. Remember, Traditional medical society threw Edward Jenner into an insane asylum for putting forth his “germ theory “. We have a history of demanding conformity, at tremendous costs to society.
I wasn’t ready for a bacteria (H. pylori) to cause peptic ulcer disease either.
Did anyone do a metaanalysis of COVID patients with their use of SSRIs?
There were many reports of housemates, spouses in particular, who did not get COVID while the other spouse was home sick with it. It would be interesting to see what other meds the well spouse might have been on at the time. See if there is a common theme
Dear Paul, thanks for this nice overview! I have two questions regarding the dosage.
1. in the earlier small randomized trial, the patients in the fluvoxamine arm received 3 x 100mg per day during 15 days. In the TOGETHER trial they received 2 x 100mg per day during 10 days. Could this have made a difference? In the earlier trial, the effect looks indeed stronger (though admittedly the sample size was very small)
2. In the routine use of fluvoxamine for depression or OCD, the dose is usually low at the beginning and then uptitrated to a max of 3 x 100mg per day. Is there no risk for more adverse events when the starting dose is immediately 2 or 3 x 100mg/day especially in frail elderly?
Looking forward to your answers.
Kind regards,
Lieven
Educated guess. Most SSRIs are developed as spin off’s from anti-histamine’s and the peak of the SSRI’s was the late 90’s with everyone “Listening to Prozac” and it seems to be a “cure-all” and every pharmaceutical company with “respect” for their shareholders and “bottom-line where pushing a “happy-pill”. Sometimes both reuptake inbitors serotonin and other mono-amines.
If I am not incorrect companies like Lundbeck pharma (A Danish company) made many of these drugs (including fluvoxamine, citalopram etc) The name fluvoxamine had a interesting story behind ( a mix of luz (ligth) vox (voice), so its a wordplay by good marketers so I guess it from Latin it would translate to “a brigth white, “God”-ligth” so shine some light into the dark mind.”
And SSRI’s are the selected based on their affinity or not for histamine receptors H1, H2, H3, anti-cholinergic (muscarin receptor a source of potential side effects blurred vision, dry mouth, constipation etc). Also for monoamine oxidase A and or B effects and so on.
Now serotonin also is very vasoactive peptide (found in the gut as well and related to bradykinin both end products of tryptophan metabolisme). And one early signs of COVID is the very red and “injected sclera’s”.
That is probably due to flush/release of many mono-amines (serotonin, bradykinin, histamine etc) and sometimes flushing of the face.
Also with for example appenditcitis facial flush is one feature to be noted (as the organ injury with appenditcitis cause big release of serotonin etc that is found richly in the gut.
And I remember red eyes (dark eyes) is a common feature with COVID. Take look at ie Trump when I he knew he had Covid-19 and how red/dark his eyes where (before he went to Walter-Reed).
SSRI tend to pro-long bleeding time as they inhibit platelets.
So given the platelet activation with COVID. How the bardykinin/serotonin/histamine pathway seems to be overactivated in the early part of the diseases, it may be plausible thay blocking this cascade makes good sense.
Some other antihistamines like ebastine is also furin/papain protease inhbitor. Famotidine another anti-histamine.
So why not blocking a surge of serotonin/histamine/chymotrypsin/papain like protease (furin) could be good strategy (as well as reducing platelet activation and vasodilatation (end keeping endothelial integrity) a important part to protect the host and reduce the virulence of the virus a good/smart strategy. According to docking/in-silico studies ebastine (an anti histamine) and potent papain like inhibitor also a harmless and potentially protective strategy.
Drugs with to narrow MOA like remdesivir seems to lack many of these good “of target” benefits.
So probably a very useful and forgotten “toolbox” is all these old SSRI’s and antihistamines that seems to be hijacked early on in the infection.
This was written without looking up any specific reference so there maybe be some small inaccuaries but this is my working/big picture understanding of the reason why this strategy may play a role.
PS: if we look back and remember the first brave Chinese ophalmologist, Dr Li W, that tried to warn about SARS-COV2 and had seen some patients (with injected sclera/red eyes/conjunctivitis, I assume). So the red eyes seems to be early sign. Pattern recognition, why would someone (more than one) go to see a ophlamologist for a pneumonia? Red eyes? Unfortunately Dr. Li died of the new infectious syndrome he probably was one of the first once to recognize, leaving a daugther and wife behind.
Well so the histamine/serotonin/bradykinin release may be the first sign something is very wrong, and why it make sense to try to stop that cascade.
The mitochondrial Sigma 1 receptor is a interesting MOA that we probably will more about soon.
I think it has been a necessary look at what was assumed to be finished and belong to the past, who would think fluvoxamine would shine again.