An ongoing dialogue on HIV/AIDS, infectious diseases,
September 20th, 2015
EHR and Drug Prescribing Warnings: The Good, the Bad, the Ugly
Recently, an ENT colleague (fictionally named “Clint” below), sent me two emails triggered by drug-drug interaction warnings he received while seeing HIV patients.
Here’s #1:
Hey Paul, I saw Mark C yesterday for hoarseness, and his exam was negative. Thought we’d try a PPI for reflux, but when I wrote the script, I got a warning that it interacted with Complera. Is this a real interaction?
Thanks,
Clint
And #2:
Paul, can’t believe I’m emailing you again. Same sort of question, different patient. Is there really an interaction between fluticasone nasal inhaler and ritonavir?
C
The answer, of course, is absolutely yes to both queries — these are very much “real” interactions, highly clinically significant. Rilpivirine (part of Complera) needs stomach acidity for adequate absorption. And the metabolism of fluticasone (and most other inhaled, injected, or even topical steroids) is blocked by ritonavir, raising systemic levels of the steroid and causing hypercortisolism — a very serious problem.
Good job, EHR! This is exactly what we want you to do, improve patient safety.
Part 2: The Bad.
But often the drug warnings aren’t really clinically relevant, and you just have to override (some would say “ignore”) them — which is why Clint the ENT (who, for the record, had never emailed me before) asked if these were “real” interactions.
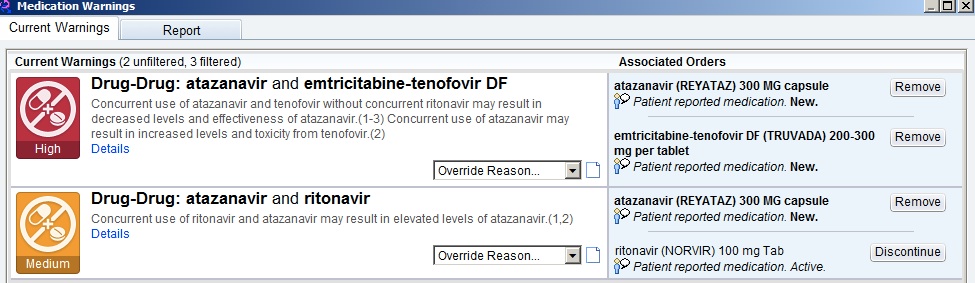
Here’s a common example every HIV/ID provider will recognize — the patient who has been receiving TDF/FTC, atazanavir, and ritonavir for years, is doing great, and needs a refill. Up comes the following:
The first one, with “high” importance, warns of the drug-lowering effects of tenofovir on atazanavir, decreasing its effectiveness — if given “without concurrent ritonavir.” (Emphasis mine.)
Hey, EHR — can’t you tell that the patient is receiving “concurrent ritonavir”? Certainly you’d think it were smart enough to do this, as the next warning, of “medium” importance, tells you that ritonavir increases atazanavir levels — exactly what we want when we give atazanavir with tenofovir. Just check out the atazanavir package insert and all the HIV treatment guidelines.
So practically we ignore both warnings, “high” and “medium” importance notwithstanding.
With warning messages like these, I suspect the following is going on: 1) No one has taken the time to teach the EHR that the complete regimen of tenofovir/FTC, atazanavir, and ritonavir should cancel these warnings; 2) the EHR doesn’t have the internal logic to check for multi-way interactions (the program generating the top-line “high” importance warning can’t read the “medium” one); or 3) some combination of the above, lost in a tangle of computer code and overwhelmed support staff.
Bad job, EHR! If warnings become too frequent, or are clinically irrelevant, this will generate “alarm fatigue.” A clinician becomes so overwhelmed by the number of warnings that he or she inevitably starts ignoring even the important ones.
Alarm fatigue is emphatically not just a problem for ICUs with their interminable beeps and buzzes, but also for EHRs. I know several primary care doctors who say they virtually always ignore them (especially on their younger patients), and housestaff entering orders on admitted patients routinely complain these alerts slow down their work, so they learn to click right through them.
If one were feeling generous, you could argue that with this “bad” example, it’s better for the EHR to err on the side of excessive caution, especially since these drug-drug interactions do exist and are at times clinically relevant — just not in this case. This problem should eventually sort itself out with greater human oversight and EHR sophistication.
(I’m an optimist.)
Part 3: The Ugly.
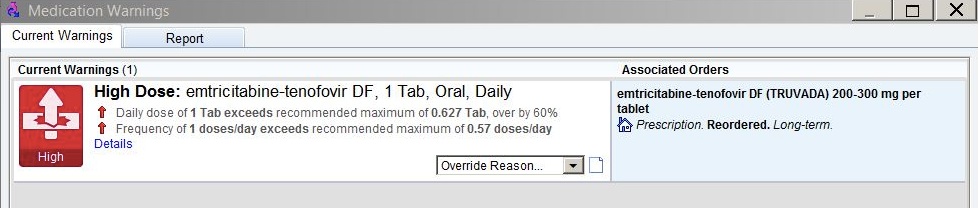
Finally, sometimes the EHR alerts are just baffling. This occurred last week as I was renewing tenofovir/FTC for a patient who, for the record, has been receiving the medication for years, has normal renal function, and normal weight.
For those of you who don’t prescribe it, the recommended dose is one tablet daily of the fixed-dose combination (only one form exists).
Up came this alert:
What the …. is going on here? I’ve prescribed this medication hundreds (maybe thousands) of times, and have never seen anything like it.
On what planet is the recommended maximum dose of tenofovir/FTC 0.627 tablets a day? Or the maximum frequency 0.57 doses a day? What does 0.57 doses a day even mean?
To make sure I’m wasn’t missing something, I’ve had two smart PharmD’s review my order, and they too are perplexed.
I reported the bogus alert, so right now, somewhere in EHR support land, a group is huddling (at least I hope they’re huddling) to try and figure out what generated this bizarre warning — one, of course, that I ignored. Or more accurately, a warning I overrode by telling the EHR that the “benefit outweighs risk” when I completed the prescription.
In short, Ugly EHR! And of course with this final example, this legendary quotation comes to mind:
To Err is Human; To Really Foul Things Up Requires a Computer
Great music here, even if you’re not a fan of Westerns:
[youtube http://www.youtube.com/watch?v=WCN5JJY_wiA&w=560&h=315]



Yes, EHR drug interaction alarms can be maddening. I do evaluate each one carefully, just to be sure there isn’t something I’m missing or unaware of. But your crazy tenofovir/FTC warning is a great example of how alarm fatigue might set in. I wind up staring at the screen and saying, “REALLY? Are you SERIOUS?” to the computer. Which I am sure makes me look like a loon.
My question is about the fluticasone nasal spray and ritonavir interaction. How clinically significant is that? 2 sprays in each nostril once a day delivers a total of 200 micrograms of fluticasone propionate. The drug primarily acts locally and has very little systemic absorption. Even accounting for its eventual metabolism, how significant is the interaction? I would be likely to override that quickly, because all the EMR knows is that I have prescribed a corticosteroid and it can’t differentiate between a nasal spray and something like oral prednisone, which *would* create a significant interaction with ritonavir. Thoughts?
Hi Loretta,
Indeed this inhaled steroid/ritonavir interaction is quite significant. Right now this is by my account the most common non-diabetes endocrinologic problem in HIV patients, and it’s iatrogenic! If you don’t believe my anecdotal opinion, here’s one good early paper, and here’s a whole slew of them!
Thanks as always for your comments/questions.
Paul
Paul
Thank you, Paul. That is news I can use. And I’ll bet a lot of other primary care providers, too. Thanks so much for the links. Although most of the articles were about fluticasone inhaled via inhaler, there were a few reports specifically about fluticasone nasal spray. Distressing that some of the patients developed HPA axis suppression so quickly. That’s one interaction I won’t override, even for short-term use! Worrisome is the fact that fluticasone nasal spray is now available OTC. I checked the Drug Facts label online, and there is a warning about ritonavir and other HIV drugs, advising the patient to check with their doctor before using fluticasone nasal. Of course, the presence of the warning and it actually being read are two different things. Nasacort/triamcinolone is also available OTC; a moderate interaction with ritonavir is reported, but there is no warning on the package.
Again, thanks so much, Paul.
Loretta, appears that beclomethasone is safest, fyi.
Paul,
I could not figure out why my patients were getting Ritonavir Gel Caps…turns out that if the pharmacy requests an inactive drug the EHR we have does not alert us to that fact and simply allows a refill of an inactive or stopped drug.
So now when I get a refill request I need to close the first pop-up screen to review the actual med list to be sure the request is not for any stopped meds before refilling.
Then I need to click thru all the warnings…we have a great one that pops up at random warning us of Bee Sting Allergy…then refill the Rx. (FWIW our EHR has NO warning about either statins or corticosteroids and ritonavir)
We alerted the EHR company to this serious safety issue la few years ago and to date no fix.
Our dumb EHR doesn’t warn us/try to stop us when we prescribe a medication that is on the patient’s allergy list! How crazy is THAT? We get all kinds of other warnings and interactions, down to possible shared tablet coloring agents (like FD&C Red 40). But if a patient is allergic to levofloxacin and I enter an Rx for levofloxacin, I don’t get any warning. That is one time when a big red stop sign would be appropriate. I was shocked when I discovered this, and now have to manually check the list of drug allergies before prescribing. This problem was reported years ago, and we are waiting for a fix, just like you are. Crazy!
The warning that I have a hard time with is one for statins. I know simvastatin should not be used but when I order atorvastatin for a patient on atazanavir and ritonavir I get a double warning telling me I’m going to give the patient rhabdomyolysis and I should keep the dose low. Some of my patients have pretty high cholesterol and 10 or 20 mg of atorvastatin is not really adequate to control the cholesterol. Is this a legitimate warning or is it okay to put patients on as much his 40 mg of atorvastatin if they are on a ritonavir boosted protease inhibitor.
Regarding the warnings for fluticasone and ritonavir let me second what you said. A number of years ago, when our LMR system only had an easy to ignore warning (just a little red blurb up in the corner of the record) I had a patient who was on fluticasone and ritonavir, she developed Cushing syndrome which took me a while to diagnose as the usual tests for Cushing’s syndrome were negative because her cortisol levels were not elevated. When with the assistance of endocrine the diagnosis was finally made she developed adrenal insufficiency when the fluticasone was stopped and needed to be on replacement for a while, so I can attest that this is a very real problem. I think it something we should all be on the lookout for because fluticasone is now available over-the-counter and also a lot of primary care doctor’s who may not be aware of this interaction prescribe fluticasone either in a asthma inhaler or nasal inhaler.
Victor,
Ritonavir increases atorvastatin levels 2-4 fold. I’d go no higher than 40 mg (and that’s what I tell our cardiologists).
Paul
I would like to add that the Truvada warning is a renal warning based on the recommendation to increase the interval to to longer than q24 hours (ie 1 tablet every 48 hours). Since the calculations are confusing to end users, the alert has been modified. Our institution has edited over 9,000 alerts that come from First Data Bank in Epic to reduce alert fatigue and this is a perfect example of one of these customizations.
Thanks for your response Kim. Actually, they’re more than just confusing — they’re wrong. As noted above, this patient has normal renal function; furthermore, there is no recommendation to give part of a Truvada tablet.