An ongoing dialogue on HIV/AIDS, infectious diseases,
March 10th, 2019
Really Rapid Review — CROI 2019 Seattle
As a foot of wet snow bore down on Boston last week — see this post for why that matters — HIV researchers and policy makers headed to Seattle for this year’s Conference on Retroviruses and Opportunistic Infections, or CROI, which took place from March 4-7.
And already I was feeling the pressure, based on this one little nudge:
Looking forward to reading @PaulSaxMD Rapid review of #CROI2019
— Aishalton (@aishalton) March 3, 2019
OK, @Aishalton, here you go with some of the highlights. And it’s not just a rapid review, but a Really Rapid Review®!
I can never cover everything, so to all brilliant readers — please use the comments section for things I’ve missed, general comments, and questions.
- Tony Fauci led off the conference with the plan to eliminate HIV in the United States. Scientifically, it’s not rocket science or brain surgery — get enough people with HIV on treatment and those at risk on pre-exposure prophylaxis (PrEP), and incidence will fall, as it has in San Francisco, New York, Australia, London, and other regions with high ART and PrEP coverage. But the big challenge in our great big and highly diverse country is that those hardest-hit with HIV — poor, minority populations, especially in the South — are exactly those with less access to medical care. Medicaid expansion, anyone? Tossing my slight skepticism aside, I noted that Dr. Fauci was genuinely excited about having all the various government groups behind it, which should eliminate some barriers. And he didn’t mention the President by name even once, proving he knows his audience!
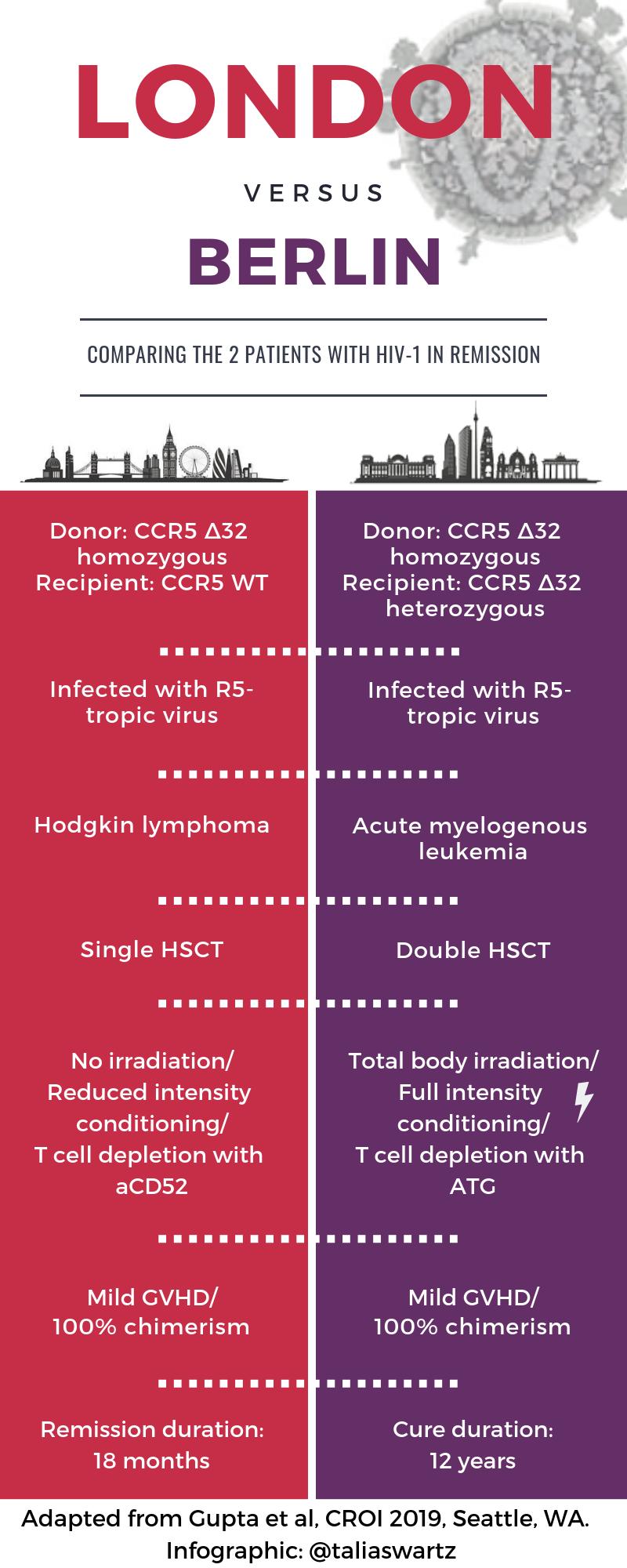
- A second person has been “cured” of HIV after receiving a stem cell transplant from a donor with the CCR5 delta 32 mutation [29]. You might have heard something about this case, ha ha ha. The transplant took place in London in a patient with refractory Hodgkin’s disease, and I put the word “cure” in quotes since there have been previous long-term remissions that weren’t durable (Mississippi Baby, Boston transplant patients). Note the important differences from the prior “Berlin” case — see awesome graphic courtesy Dr. Talia Swartz. And no, it’s still not a viable strategy for broader application, for clear reasons nicely articulated in this piece by Gregg Gonsalves — but an n of 2 is 100% better than an n of 1!
- But could it be an n of 3 [394]? This patient from Düsseldorf (why are all these cases in Europe?) has been off treatment since November 2018 — stay tuned. Remember, other stem cell transplants from CCR5-negative donors have not achieved remission, such as this notable example.
- For HIV pre-exposure prophylaxis, TAF/FTC was noninferior to TDF/FTC [104]. Despite high rates of sexually transmitted infections (STIs) in the study population, demonstrating ongoing potentially high-risk contacts, the incidence of HIV infection in this large study was much lower than expected. Hence, the results met the noninferiority threshold only because there were numerically fewer infections in the TAF/FTC vs. TDF/FTC group (7 vs. 15). Concerns about low incidence notwithstanding, the results argue that TAF/FTC is at least not too much worse (“noninferior” in English) in preventing HIV than TDF/FTC. In my opinion, it would now be preferred if there is pre-existing renal or bone disease.
- An electronic medical record algorithm can identify the best candidates for PrEP [105]. It is unrealistic to expect primary care clinicians to consider PrEP for all the patients in their panel, as the vast majority are not at high enough risk for acquiring HIV. In this study from Kaiser California, the top 1% HIV risk score generated by the EMR algorithm identified over 6000 candidates — with 92% not receiving PrEP. Seems like an excellent strategy to bring PrEP to those who need it the most.
- Among over 1437 people attending a New York City sexual health clinic, 97% started PrEP immediately after a clinical screen for acute HIV, hepatitis B, or impaired renal function [962]. The only test back before starting PrEP was a negative rapid HIV test. The study is part a laudable effort in New York to reduce barriers to PrEP initiation.
- In Atlanta, young black MSM who started PrEP had a high likelihood of stopping [963]. Roughly 125 started PrEP in a dedicated program for this patient population. During 720 days of follow-up, 63% stopped at least once, and 31% stopped completely.
- Out of 3685 patients newly diagnosed with HIV over a 2-year period in NYC, only 91 (2%) had been PrEP users — and nearly a third of them had the M184V mutation [107]. No K65R mutations were detected. By contrast, M184V was found in 2% of those who never used PrEP. Remarkably, only 75% of those who acquired HIV on PrEP had baseline genotypes! Seems like taking PrEP and then getting HIV infection is an emphatically good indication for clinicians to obtain resistance testing.
- A randomized population-based effort to provide ART to highly prevalent communities in Southern Africa reduced HIV incidence, but not in all study arms [92]. The trial, called POPART, randomized populations to standard-of-care, universal ART, or ART provided based on CD4 thresholds (initially 350, then 500, then universal). Surprisingly, only the last of these significantly reduced incidence. The results imply that even with 90-90-90 targets met, there is sufficient ongoing transmission that treatment alone is unlikely to end the epidemic in our highest prevalence areas.
- In patients with long-term viral suppression and no resistance, monthly, long-acting, injectable cabotegravir and rilpivirine was noninferior to continued oral therapy [139]. The median time of suppression prior to switch was 4 years. Only 1.6% and 1.0% had viral loads >50 at week 48 in the injectable and oral therapy arms, respectively. Injection-site reactions were common, but mostly mild. Patient satisfaction scores for the injectable strategy were off-the-charts favorable. (FYI, the study is called ATLAS, which I’m sure stands for something clever.)
- After treatment-naive patients started treatment with ABC/3TC/DTG for 20 weeks and achieved viral suppression, monthly, long-acting, injectable cabotegravir and rilpivirine was noninferior to continued ABC/3TC/DTG [140]. In this second study (called FLAIR), 2.1% and 2.5% had week 48 viral loads >50 in the injectable and ABC/3TC/DTG arms, respectively, at week 48. The tolerability of the injections and the treatment satisfaction scores? Again, excellent.
- In both ATLAS and FLAIR, a small number of participants failed the injectable therapy with emergent drug resistance despite adhering to study visits. Three such cases occurred in each study. For reasons that are still unclear (at least to me), 5/6 were enrolled in Russia, and all 5 harbored HIV subtype A1. In the FLAIR study, two patients developed the Q148R integrase-resistance mutation. Drug levels in all these participants were in the lower end of the distribution, but well within the range in which other study subjects maintained suppression. A mystery! Webcasts here and here.
- In treatment-experienced patients failing first-line therapy with 2 NRTIs/1 NNRTI, dolutegravir (+ NRTIs) is superior to lopinavir/r (+ NRTIs) regardless of baseline NRTI resistance [144]. Important, confirmatory results, with the full study recently published here. Based on the study design (which mandated that all study participants receive at least one genotypically active NRTI), the results cannot tell us the preferred NRTI combination to use with the baseline K65R plus M184V mutations — which didn’t stop me from asking about this after the presentation. I thought perhaps after looking at all those genotype results, the answer would magically emerge to the investigators!
- Transmitted drug resistance in the USA remains essentially stable [526]. The key percentages resistant by class: non-nucleoside reverse transcriptase inhibitors (NNRTIs) (11.9%), nucleoside reverse transcriptase inhibitors (NRTIs) (6.8%), protease inhibitors (PIs) (4.3%), and INSTIs (0.8%). These numbers represent small increases in NNRTI and INSTI resistance, but at this point hardly enough to change practice. (Hey, did you know that the CDC does these studies off of resistance genotypes that are ordered in clinical care and then forwarded to them? Seems like something that should be done with a more representative and population-based approach.)
- Providing viral load results during clinic visits significantly improved viral suppression and retention in care [53LB]. Investigators in South Africa randomized 390 people at their month 6 follow-up visit after starting ART to point-of-care viral load results (given to patients at their visit) or usual lab-based testing. Twelve months later, viral suppression was observed in 89.7% of the point-of-care group, vs 75.9% in the standard of care arm, a whopping 14% difference. If there were differences this profound in a clinical trial comparing HIV treatments, a DSMB would stop the study early!
- Several studies implicated integrase inhibitors (INSTIs) as treatment-related contributors to excess weight gain. Abstracts 669 (switch), 670 (initiation), and 672 (switch in women) all found a positive signal; abstract 671 (switch) did not, once controlling for other factors. (And weight gain is the very definition of multifactorial!) Neither did cabotegravir in a small study of people without HIV [34]. However, since a randomized clinical trial of switching from PI-based to DTG-based regimen demonstrated significant weight gain with the switch, this association with INSTIs appears to be causal.
- Baseline NRTI resistance did not influence viral suppression in patients successfully treated with tenofovir/FTC plus DTG if they were randomized to switch to bictegravir/FTC/TAF [551]. This is an interim analysis of an ongoing clinical trial, suggesting that as with treatment-naive patients and those switching with no resistance, DTG and BIC-based regimens are very similar. Note that “archive” DNA resistance testing identified resistance mutations not seen on historical genotypes; the opposite also occurred. Hmmm … wonder which is the gold standard?
- An investigational capsid inhibitor provided potentially therapeutic drug levels for 12 weeks after a single subcutaneous injection [141]. Phase 1 studies in people with HIV have begun. Key for this long-acting, potent agent (GS-6207) will be to find an appropriate partner drug.
- A single infusion of a broadly neutralizing antibody (bNAb) PGT121 sustained viral control for 6 months in two patients with low baseline viral loads [145]. By contrast, among the nine treated participants with viral loads higher than 3.3 log, four of nine did not respond at all. Several other presentations about bNAbs at the conference (here and here, for example), but this line of therapy is still in its infancy. (Two questions — does some savant know the names all of these bNAbs? Why isn’t “bNAb” spelled “BNAb,” with the “B” capitalized?)
- More studies aiming to improve the abnormal inflammatory or heightened immune activation status of people with HIV demonstrated no clear benefits. For the record, the interventions at this CROI included sirolimus, probiotics, and ruxolitinib. Hey folks — the immune system is complicated! It would not surprise me if further interventions in this area are limited to those treatments that would be indicated otherwise — e.g., statins for reducing cardiovascular disease.
- Opioid-overdose deaths among people with HIV have significantly increased since 2011 [147]. The increase has occurred in all transmission groups, not only those who acquired HIV via injection drugs.
- Ledipasvir/sofosbuvir successfully treated HCV in a small number of pregnant women [87]. Only 8 women were eligible; 10 potential participants were excluded due to genotype 2 or 3 infection. All had acquired HCV from injection drug use. The results suggest the next study should be with velpatasvir/sofosbuvir or glecaprevir/pibrentasvir — and also point to the huge data gap of HCV treatment in this population, as this is the only prospective study in pregnancy to date!
- Women late in pregnancy (>28 weeks gestation) and not on ART experienced a significantly shorter time to viral suppression when randomized to DTG vs EFV-based regimens [40]. Roughly 74% in the DTG arm and 43% in the EFV arm had viral loads <50 at delivery, a highly significant difference. These results appear to favor DTG, with two caveats that
 might not be drug related — three HIV transmissions and four stillbirths occurred, all in the DTG arm. Transmissions were believed to be in utero transmissions — remember that starting ART late in pregnancy is associated with a 7-fold increased risk of maternal-to-child transmission, and a doubling of infant mortality in the first year. Name of this study? DolPHIN-2 (DOLutegravir in Pregnant HIV Mothers and TheIr Neonates) — and not to be confused with DolPHIN1— or the next study.
might not be drug related — three HIV transmissions and four stillbirths occurred, all in the DTG arm. Transmissions were believed to be in utero transmissions — remember that starting ART late in pregnancy is associated with a 7-fold increased risk of maternal-to-child transmission, and a doubling of infant mortality in the first year. Name of this study? DolPHIN-2 (DOLutegravir in Pregnant HIV Mothers and TheIr Neonates) — and not to be confused with DolPHIN1— or the next study. - Dolutegravir levels were reduced when given with preventive therapy of weekly isoniazid and rifapentine, but not enough to warrant dose adjustment [80]. Viral suppression was maintained, with no significant adverse events. Importantly, for treatment of TB (with daily rifampin), dolutegravir dosing should be changed to 50 mg twice daily. Name of this study? Also “DOLPHIN”, for DOLutegravir, rifaPentine, INH, INvestigation. (Notice how I put those together — clever, eh? And what are the chances of a single CROI having two completely unrelated HIV-related studies named “DOLPHIN”?)
- Cannabis appeared to improve blood brain barrier injury and CNS inflammation [459]. Of course it did! We were in Seattle, remember? And that’s not a skunk you’re smelling.
Look, even though your Netflix beckons, if you have a few moments, the following webcasts by experts on diverse topics are must watch for any HIV/ID specialist. I’m listing below my favorites, with the caveat that I couldn’t see all the talks and might have missed some other gem:
- David Back on Pharmacology and Drug Interactions
- Jeanne Marrazzo on STIs
- Irini Sereti on Inflammation
- Jean-Michel Molina on PrEP Challenges
- Laura Waters on Two-Drug Therapy
So, what did I miss? Let me have it.


although some services offer genetic testing it is difficult to see what benefit this would give an individual. Even if someone has a genetic resistance to most HIV infections, they can still be infected by other strains. i-Base doesn’t provide links to commercial sites unless there is a therapeutic need for HIV positive people.
Paul,
Wonderful Rapid Review, as always!! Thanks so much!
One thing I would add: as you know, TAF has been shown to have low levels in female cervical and vaginal fluid/tissues, and DISCOVER did not include cis-women. Hence, although TAF/FTC looks great for MSM and TG women, I would NOT use for any cis-women at this point in time. (Unless I have missed new data of TAF use as PrEP in women??)
My question concenrs point of care viral load testing. I am not an ID doc or HIv provider. Is this currently done in the US? If not are there plans to pilot this in citirs with high prevalence in the US?
Thanks, Paul!