An ongoing dialogue on HIV/AIDS, infectious diseases,
February 19th, 2010
CROI 2011 Dates: February 27-March 3, Boston
 CROI just about wrapping up — excellent, as usual. Hope to provide some “greatest hits” shortly.
CROI just about wrapping up — excellent, as usual. Hope to provide some “greatest hits” shortly.
But since John Mellors announced the dates of next year’s conference — and because the CROI web site can be “leisurely” in posting this information — I offer the following evidence as a public service to researchers, teachers, clinicians, and schedule-makers out there in HIV/ID Land.
Come for the conference, stay for the weather!
February 14th, 2010
Retrovirus Conference (CROI) 2010 Preview
 Just as pitchers and catchers report to Spring Training this week, many HIV specialists are gearing up for the 17th Conference on Retroviruses and Opportunistic Infections (CROI), which starts this Tuesday in San Francisco.
Just as pitchers and catchers report to Spring Training this week, many HIV specialists are gearing up for the 17th Conference on Retroviruses and Opportunistic Infections (CROI), which starts this Tuesday in San Francisco.
(I don’t suppose many people see the link between those two events. Oh well.)
And since the “pocket program” to the Conference has been available since last week, below is a highly-subjective (admittedly developed-world centric) list of potentially important presentations, in a somewhat random order. Apologies ahead of time for leaving off something of great importance, most likely buried somewhere deep in the poster presentations.
- 28 Immunodeficiency, HIV RNA Levels, and Risk of Non-AIDS-defining Cancers
- 30 HIV Infection Is an Independent Risk Factor for Lung Cancer
- 33 Decreases in Community Viral Load Are Associated with a Reduction in New HIV Diagnoses in San Francisco
- 34 Monitoring the Impact of Expanded HIV Testing in the District of Columbia Using Population-based HIV/AIDS Surveillance Data
- 53 Safety and Efficacy of TBR 652, a CCR5 Antagonist, in HIV-1-infected, ART-experienced, CCR5 Antagonist–Naïve Patients
- 54LB Phase 3 Trials of Vicriviroc in Treatment-experienced Subjects Demonstrate Safety but Not Significantly Superior Efficacy over Potent Background Regimens Alone
- 57 Efficacy and Safety at 48 Weeks of Once-daily vs Twice-daily DRV/r in Treatment-experienced HIV-1+ Patients with No DRV Resistance-associated Mutations: The ODIN Trial
- 58LB Single-tablet, Fixed-dose Regimen of Elvitegravir/Emtricitabine/Tenofovir Disoproxil Fumarate/GS-9350 Achieves a High Rate of Virologic Suppression and GS-9350 Is an Effective Booster
February 11th, 2010
Ritonavir Tablet Approved
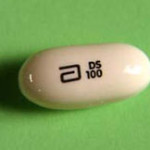 It’s not on Abbott’s web site yet (update: now it’s here), but the FDA has approved a new formulation of ritonavir — a heat-stable 100 mg tablet. From an e-mail release by the FDA:
It’s not on Abbott’s web site yet (update: now it’s here), but the FDA has approved a new formulation of ritonavir — a heat-stable 100 mg tablet. From an e-mail release by the FDA:
On February 10, 2010, FDA approved Norvir (ritonavir) 100 mg Tablets. These tablets do not require refrigeration. Unlike the capsule formulation [pictured], Norvir tablets must be taken with meals.
–snip–
NORVIR tablets are not bioequivalent to NORVIR capsules. Under moderate fat conditions (857 kcal; 31% fat, 13% protein, 56% carbohydrates), when a single 100 mg NORVIR dose was administered as a tablet compared with a capsule, AUC(0- ∞) met equivalence criteria but mean Cmax was increased by 26% (92.8% confidence intervals: ↑15 -↑39%).
This is obviously an advance — the Norvir soft-gel capsule is much-despised for a variety of reasons, one of the most notable being the glob of melted capsules that results if the medication is left in the car, in a warm room in the summertime, near a radiator, etc. When Kaletra switched from capsule to tablet, the heat-stability was a big improvement in convenience, and also made treatment in resource-limited settings much easier.
Time will tell if the increase in peak levels has any effect on tolerability, a potential concern since much of ritonavir’s toxicity is dose-related. I suspect Abbott will have both formulations available for a while until this is sorted out.
And for those who still have patients still on 600 mg twice-daily — it’s time to switch to something else!
February 8th, 2010
Is She or Isn’t She …
… taking antiretrovirals?
Over on our Journal Watch/AIDS Clinical Care web site, we’ve posted case of a pregnant woman who says she’s taking ART as prescribed, but the lab tests say otherwise.
How do you manage these cases?
February 4th, 2010
Non-Cirrhotic Portal Hypertension: A Rare but Serious Side Effect of ddI
 The FDA has issued a warning about an association between use of ddI (didanosine) and the development of non-cirrhotic portal hypertension:
The FDA has issued a warning about an association between use of ddI (didanosine) and the development of non-cirrhotic portal hypertension:
Non-cirrhotic portal hypertension (portal hypertension that is not caused by cirrhosis of the liver) is rare in the United States. It occurs when blood flow in the major vein in the liver (the portal vein) slows down.
–snip–
Because of the potential severity of portal hypertension, including death from hemorrhaging esophageal varices, FDA has revised the Warning and Precautions section of the didanosine drug label to assure safe use of the medication.
Yes, this is a bad one — of the 42 reported cases, 4 patients died; several others required aggressive interventions to reduce the risk of variceal bleeding, including banding/ligation of esophageal varices, transjugular intrahepatic portosystemic shunts (TIPPS), and liver transplantation.
ddI was our second approved antiretroviral, way back in 1991.
Over the years, it has improved — we’ve reduced the dose, now can give it once daily, and have gotten rid of the original bizarre formulation (pictured).
And while it does have reasonable antiviral potency (at least for an NRTI), its myriad potentially severe side effects — neuropathy, pancreatitis, lactic acidosis, to name a few — and the availability of numerous other options mean that there is little reason for a person to be receiving it today.
February 2nd, 2010
Bats in the Bedroom: Canadians Make a Policy Change
 ID doctors know all too well the panicky call — usually from a terrified friend, family member, or colleague, or possibly the emergency room or primary care doc — about finding a bat in the house.
ID doctors know all too well the panicky call — usually from a terrified friend, family member, or colleague, or possibly the emergency room or primary care doc — about finding a bat in the house.
Usually in the bedroom.
(In one memorable case, it was the house cat’s leaping in the air to try and catch the bat that woke up the sleeping couple. Each missed pounce led to a loud “thud” as the cat fell to the ground.)
The concern, of course, is bat-related rabies, which I’ve written about before, mostly at the prompting of a fine pair of papers (summarized here and here) published over the past few years by a Canadian group. They have painstakingly documented just how rare human rabies arises from “stealth” bat exposure in the house — 1 case per 2.7 billion person-years
And number of such situations that would need rabies vaccination to prevent one case? From a low of 314,000 to whopping 2.7 million persons.
Well Canada’s National Advisory Committee on Immunization (NACI) has read these papers too, and has decided to stop recommending rabies immunization for bats-in-house-but-no-exposure cases:
Researchers have since reviewed this approach and determined that when there is no obvious contact with a bat, the risk of rabies is extremely rare. Furthermore, there are considerable resource implications to implementing this strategy. Based on the review, which is outlined below, NACI is now recommending intervention only when both of the following conditions apply: 1) There has been direct contact with a bat; and 2) A bite, scratch, or saliva exposure into a wound or mucous membrane cannot be ruled out
According to this nice summary — and hat tip to the author for notifying me of policy change in Canada — it doesn’t sound like the US Advisory Committee on Immunization Practices is ready to make the change quite yet.
But maybe in the next revision of these guidelines?
January 29th, 2010
More on TaqMan Viral Load Testing
 Since I first discussed the disruptive effect of introducing Mr. TaqMan to our clinic, many others have weighed in.
Since I first discussed the disruptive effect of introducing Mr. TaqMan to our clinic, many others have weighed in.
One of my favorite reports is a nice paper from the Alabama group, presented first at IDSA, and soon to be published. It shows not only a higher rate of low-level detectable results, but also the increased costs (substantial) due to repeat testing, resistance genotypes sent (and not yielding anything, of course), and the waste of patient and clinic time.
Today, a new complaint: the convoluted word salad that follows the results. To wit, here’s one recent lab report:
HIV-1 PCR: Detected (H)
HIV-1 PCR QUANT: 170 [yes, 6 prior values were undetectable]
HIV-1 PCR QUANT LOG: 2.23
HIV PCR Interpretation: SEE BELOWComments: The quantitative range of this assay is 48-10,000,000 copies/ml (1.7-7.0 log copies/ml). In addition to the sample result being expressed as both copies per milliliter (copies/ml) units and as log(copies/ml), this report interprets if the virus was “Detected”, “Not detected” or “Detected but below Quantitative range” as described below in more detail:
a) “Detected” – means that the viral titer in the sample is above the assay’s lower limit of quantitation (48 copies/ml);
b) “Detected – Not Quant” – means that the viral titer in the sample is below the lower limit of quantitation but above the assay’s lower limit of detection ranging from less than 15 to 46 copies ml across all subtypes of HIV-1 group M;
c) “Not Detected” – means that the viral titer in the sample is below the assay’s lower limit of detection. An interpretation of “Not Detected” does not rule out the presence of HIV-1 that is no detectable for reasons such as PCR inhibitors or HIV-1 virus RNA concentrations below the lower level of detection of the assay.
This test is intended for use in conjunction with clinical presentation and other laboratory indices of disease status as an aid in the clinical management of HIV-1 infected patients. The test can be used to assess patient prognosis by monitoring the effect of antiretroviral therapy on changes in plasma HIV-1 RNA levels during the course of treatment. Limitations: The COBAS AmpliPrep/COBAS TaqMan HIV-1 Test is not intended for use as a screening test for the presence of HIV-1 in blood or blood products or as a diagnostic test to confirm the initial presence of HIV-1infection. General Disclaimer: Interpretation of this test may be affected by the presence of rare viral RNA variants. Methodology: The COBAS AmpliPrep/COBAS TaqMan HIV-1 Test utilizes automated specimen preparation followed by automated reverse transcription, PCR amplification and detection of cleaved dual-labeled detection probes specific to the HIV-1 target RNA. One copy of HIV-1 RNA is equivalent to 1.7 +/- 0.1 International Units (IU) based on the WHO 1st International Standard for HIV-1 RNA for Nucleic Acid-Based Techniques (NAT) (NIBSC 97/656). Test Lab: This assay is approved for In Vitro Diagnostic (IVD) use by the FDA. This test is used for clinical purposes, and it should not be regarded as investigational or for research.
Yikes.
One of my colleagues has responded by going back to sending bDNA. Yes, that test might be less sensitive, but can anyone tell us yet whether these low-level detectable values have any clinical meaning?
January 17th, 2010
Hey, Didn’t You Used to be the Cause of CFS?

The report last year that xenotropic murine leukemia virus-related virus (XMRV) was found in a high proportion of patients with Chronic Fatigue Syndrome (CFS) caused quite a stir — which is totally understandable given how frustrated the people with CFS are with the lack of adequate explanations for their suffering.
The investigators of the original report even began referring patients to a commercial lab (question #5) to get tested for the virus.
Now some British researchers weigh in. Their findings:
Patients in our CFS cohort had undergone medical screening to exclude detectable organic illness and met the CDC criteria for CFS. DNA extracted from blood samples of 186 CFS patients were screened for XMRV provirus and for the closely related murine leukaemia virus by nested PCR using specific oligonucleotide primers.
(snip)
XMRV or MLV sequences were not amplified from DNA originating from CFS patients in the UK.
Oh darn.
One possible explanation for the negative finding is the differing epidemiology of XMRV in North America and Europe, something also noted in studies of XMRV and prostate cancer.
But the results certainly reinforce what I have suspected for some time, which is that CFS most likely has multiple causes — some infectious, some allergic, some environmental, some emotional, but all yielding a similar clinical phenotype due to underlying genetics and how an individual responds to illness. Yes, XMRV might cause CFS in some people — but seems highly unlikely it does so in all.
I wish it were simpler than that, but alas don’t think it will be.
(Nice summary of the controversy here in ScienceNOW.)
January 14th, 2010
Magic Wand Destroys H1N1 — and More!
 From the folks at Hammacher Schlemmer comes this extraordinary device:
From the folks at Hammacher Schlemmer comes this extraordinary device:
Tests performed by an independent antimicrobial testing laboratory showed the wand destroyed 99.98% of the H1N1 virus after a five-second exposure when held 3/4″ above the contaminated surface. Also capable of killing MRSA, mold, and dust mites, the UV-C light penetrates viral and bacterial membranes and destroys their DNA, rendering the microorganisms incapable of reproduction and survival.
It’s worth noting that this thing became available just as the number of flu cases began to plummet.
Do we have this magic wand to thank?

 Apparently, Merck — taking over for Schering-Plough —
Apparently, Merck — taking over for Schering-Plough — 