An ongoing dialogue on HIV/AIDS, infectious diseases,
July 14th, 2013
Will Dolutegravir Instantly Become the Integrase Inhibitor of Choice in Patients with Treatment Failure?
 Here’s the short answer : Yes. Probably.
Here’s the short answer : Yes. Probably.
And here’s why.
In a randomized, double blind clinical trial just published in the Lancet — it’s called SAILING — once-daily dolutegravir was compared to twice daily raltegravir in treatment-experienced patients. The site investigators could choose one or two other fully active agents to develop an optimized background regimen (OBR). There was a protocol-specified stratification based on fully-active darunavir and baseline viral load.
There were over 700 study subjects; they were recruited from 156 sites, located in every continent except Antarctica.
(Yes, 156 sites. Hey, it’s hard to find patients who fail treatment who are good candidates for clinical trials!)
And here are the results:
- Virologic outcome: Dolutegravir was superior to raltegravir, 71% suppressed at 48 weeks, vs 64%, p=0.03.
- Those experiencing virologic failure had less resistance on dolutegravir than raltegravir.
- In the hardest-to-treat subgroups — those with no active darunavir in their OBR, or the higher viral load stratum — the differences favoring dolutegravir over raltegravir were even greater than in study overall.
- Adverse events, serious or otherwise — pretty much the same.
Now I’ve said before that raltegravir is one of our very best antiretroviral agents: it’s highly potent, well tolerated, has very few drug-drug interactions, an extensive safety record dating back to clinical trials that started in the mid-2000s, and and even more extensive favorable clinical experience since its approval in 2007.
But dolutegravir is once daily, seems just as well tolerated as raltegravir, has a similar drug-drug interaction profile — and based in the results of SAILING, is probably more potent and less prone to resistance.
Note that elvitegravir isn’t currently in the discussion, since it’s not available except as part of this one pill — TDF/FTC/EVG/COBI — which can’t be given with boosted PIs, so is a difficult choice in those with treatment failure.
Bottom line: barring some unexpected toxicity when it gets into broader use after approval — always a possibility — dolutegravir will instantly become the integrase inhibitor (II, INI, INSTI, InSTI) of choice in treatment experienced patients with treatment failure.
(Still need a good abbreviation for this drug class.)
July 7th, 2013
Almost Annual Whine About No CROI Dates, and a New Temporary (I Hope) CROI Website
Believe or not, sometimes we know a year in advance the dates of the Conference on Retroviruses and Opportunistic Infections (CROI).
For example, we learned at the beginning of CROI 2010 that in 2011, it would take place February 27-March 3 — in Boston no less. Yay!
(The meeting was a bit shorter, but it did in fact start on Feb 27.)
But most of the time, “When is CROI next year?” is a free-floating mystery, one that can’t be solved until a surprisingly short time before the actual meeting.
We know that CROI will be in the winter, probably in February or March, and that abstracts will be due some time in the fall. Plus we know CROI will be held someplace in North America, though of course the location is far less important for planning academic and personal calendars than knowing the dates.
Because of this uncertainty, those of us who want to attend the meeting go through aggressive calendar-managing in order to avoid important conflicts. In what is now an annual two-month blockade, here are some typical approaches to events in February and March:
- Thinking about attending on the inpatient clinical service during those months? Bad idea.
- Want to go on a family vacation? Reconsider.
- Invited to speak in a medical school or postgraduate course, or to give medical grand rounds? Think again.
- Want to get married, or to adopt a puppy, or to sign up for that adult ed class on using power tools? Better wait until April.
When I try to explain this odd state of affairs to my non-ID colleagues, they are invariably perplexed, or say something naive like “Why don’t you check the conference website?”
That should be a good idea, so let’s do that. The conference website — retroconference.org — is in fact excellent, with a rapid search feature for abstracts, posters, and many terrific web casts.
That is, it was excellent — the site no longer works. In its place, we have this:
For the record, I have long assumed that we don’t know when CROI will be because the conference organizers don’t know yet either. (When I directly ask them, this is what they say — “We don’t know yet.”)
But if someone does know, and they’re hiding it — as in this example or this one — perhaps Edward Snowden can leak it when he lands in Bolivia, or Venezuela, or wherever he ends up.
July 3rd, 2013
First Year ID Fellows — What Do They Learn, and What Do They Hate?
In the weird calendar of academic medical centers, July 1 is the “official” first day of school.
In our ID program, however, we shifted it to July 5 a few years ago to avoid the interruption of the July 4 holiday at the beginning of the year. On July 3 — today — our incoming first-year fellows round with our ID fellows who have completed 363 days, acting as observers.
If ever you wanted tangible evidence that the first-year learning curve has a very large area under it, this is the day. Because the soon-to-be second years know a ton, the newbies … well, let’s just say there’s a reason they do an ID fellowship, and it’s to learn clinical ID!
In addition, fellows who have spent a year doing ID consults nearly every day also have learned a few things that they truly hate.
Here’s one: A late afternoon consult.
(That was easy.)
But rather than my providing the list, I would love to hear from you — two targeted questions:
- What do first-year fellows learn?
- What do they learn to hate?
Responses — anonymous and otherwise — welcome! And here’s a video:
June 27th, 2013
Testing Out the New Website with an ID Link-o-Rama
Hey, new website is live! Interested to hear what you think about our new-ish look.
In celebration, here are some quick ID/HIV tidbits that have recently crossed my path, or have been sitting in my inbox for a while, dying to get out:
- Doxycycline shortage. Hardly anything more frightening to a New England ID doc than a shortage of the world’s most versatile antibiotic during peak Lyme season. Shudder. Fortunately, most of my patients have had no trouble getting it from their pharmacies, though I do hear prices are way up.
- Some HCV news — USPSTF recommends screening baby-boomers (joining CDC, they had already recommended the same), and an HCV genotype test is approved. Here’s the question — why is the latter news at all? We’ve been sending HCV genotypes — “unapproved,” I guess — for years and years.
- More HCV: Did you see the CDC study published this Spring showing that around half the people with HCV don’t get a complete evaluation? Specifically, after a positive antibody test, many have no subsequent HCV RNA (viral load) checked to determine whether they have chronic disease. How is that possible?
- Another ID competition from John Bartlett. This time, it’s “Most Important Antiviral”. Go over to Medscape and vote and, more importantly, go read John’s fascinating discussion — which I bet he dictated between 4 and 5 a.m. early one day without using notes.
- A retrospective study says you can stop antibiotics on patients with ventilator-associated pneumonia if sputum cultures are negative. Yeah — RIGHT. (Readers of a certain age will say that with the full Bill Cosby effect.) NEJM Journal Watch summary here.
- Nice balanced piece in the New Yorker on the Lyme Disease controversy. Kudos to the author for not implying that there are villains on either side — a common mistake when the issue is covered in the lay press.
- And on the subject of ticks, this time of the Lone Star variety, how about this bizarre connection — tick bites and allergy to red meat. Note that the allergic reaction is delayed, which may make the connection difficult to diagnose. The article includes the inevitable advice about wearing long pants and tucking them into your socks during the summer. Again — RIGHT. Perhaps no public health advice is less heeded than that one, beating out even condom use. (Love the interview at the start of that video, by the way — a young couple doing condom testing for “science”!)
- The Kaiser Family Foundation has released this fascinating report on the funding sources for global health related to HIV/AIDS. Lots of interesting data, including the fact that the U.S. provides almost two thirds of all HIV/AIDS international assistance (61%), with the next largest donor, the Global Fund, providing one fifth — that’s around 80% of the total, if I’ve done my math right.
- Wouldn’t it be nice to have something that “eliminates up to 99.9% of airborne bacteria, mold, viruses”? And now, for just $299.95, it can be yours. Good thing it isn’t $300.00, that would be way too expensive.
By the way, wondering how to say “NEJM Group”? It’s N-E-J-M Group, spelled out, and not preceded by the word “The.” Now we all know. And I guess the Mass Medical Society prefers their doctors to be photographed wearing a tie.
Enjoy this classic comedy!
June 22nd, 2013
NEJM Group, Blog Freeze, and Puppy Brothers
It’s official — the various medical products of the Massachusetts Medical Society are now lumped under NEJM Group — including the flagship New England Journal of Medicine itself, the Journal Watches, even this site.
No surprise, therefore, that there will be a new web platform, with the launch slated for next week. I’ve been told this means a “blog freeze” — sounds like a summer ice cream treat — for at least June 24-25. During the freeze, there will be no new posts or comments.
Here then, to bide the time until we’re up and running again, is a picture of our puppy Louie and his brother Arlo, who fortunately just happens to live down the street:
June 20th, 2013
Let’s Move the HIV Testing Algorithm Into the 21st Century
As I’ve written before, the most widely used testing algorithm for HIV — enzyme immunoassay followed, if positive, by Western blot confirmation — is long overdue for an update.
A brief review why this is the case, and also why sticking with it is so problematic:
- Immunoassays have become progressively more sensitive, especially when paired with p24 antigen testing in “4th generation” tests.
- In recently acquired infection, the screening test turns positive way before the Western blot — it can be a difference of several weeks (figure from Branson J Acquir Immune Defic Syndr 2010;55:S102–S105):
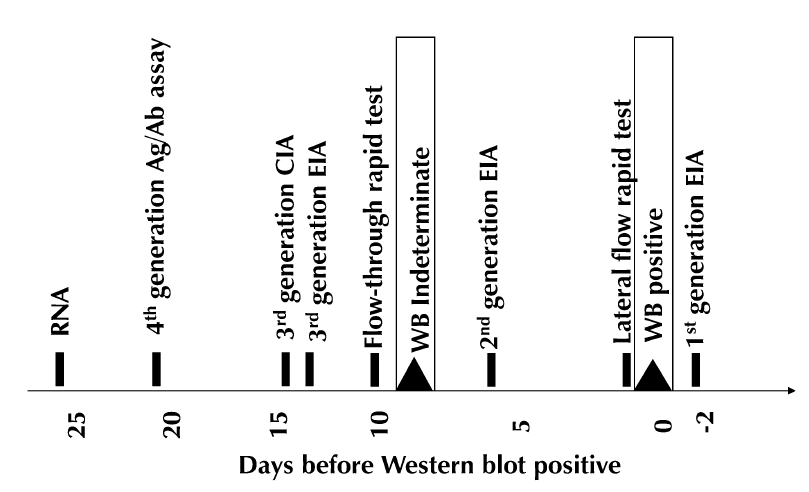
- Clinicians are not familiar with this problem, so they might conclude that a reactive screening test followed by a negative Western blot means that the person doesn’t have HIV — it’s a “confirmatory test”, after all, so you can understand their confusion.
- Patients during this phase of early/acute HIV infection are at their most contagious, hence most likely to spread the virus to others — especially if given the wrong information about their HIV status.
- Patients may seek testing soon after high-risk exposures — they’re motivated! — and hence are potentially in this window before the Western blot turns positive.
The frustrating thing about this state of affairs is that we have had the tools to correct this problem for some time. Laboratory guidelines for HIV testing were updated in 2011, recommending the following algorithm for HIV diagnosis:
Now, thanks to CDC, we have some concrete data about how useful this new testing strategy can be — in particular for those with recently acquired HIV. A screening program in an Arizona emergency department identified 37 individuals with undiagnosed with HIV, and twelve of them — nearly a third — had acute HIV that otherwise would have been missed by the Western blot.
A second validation study looked at over 37,000 samples drawn from high-risk patients in sites from New York City, North Carolina, and San Francisco; there were 99 cases where the screening immunoassay was positive and the second antibody test negative. In this group, 55 had acute HIV infection (diagnosed by HIV RNA), many of whom had negative Western blots.
According to Bernie Branson — HIV testing guru from CDC — the FDA approval of the differentiation assay (step 2 in the algorithm) has led many labs to adopt the new strategy:
The number of labs doing this has picked up considerably since Multispot received its new indication … nearly half of public health labs (e.g., FL, MA, IL, IA, NY) have adopted it, as have Stroger/Cook County in Chicago, Howard University Hospital in DC, and LSU hospital system (to name a few). California has proposed emergency regulations (expected to be effective July 2) that will permit all labs in the state to perform the new algorithm, which their current regulation precludes, and New York State has issued 2 public health advisories encouraging its use.
It’s time for the rest of us to do the same.
And if you’re feeling bad for the Western blot, it still has a (rare) indication — as a confirmation of HIV infection in “elite controllers”, those with HIV antibodies but no detectable HIV RNA.
June 19th, 2013
FDA, IND, FMT: Nine Letters, Some Common Sense, and a Real Video Link
Good news here — the FDA has reconsidered their requirement for an IND for fecal microbiota transplantation (FMT) for C diff:
Some health care providers have stated that applying IND requirements will make FMT unavailable and have suggested that an alternative regulatory approach is needed to ensure the widespread availability of FMT for individuals with C. difficile infection unresponsive to standard therapies. The agency acknowledges these concerns and intends to exercise enforcement discretion regarding the IND requirements for the use of FMT to treat C. difficile infection not responding to standard therapies provided the treating physician obtains adequate informed consent from the patient.
This approach certainly makes sense.
Still, on reading the statement, one might wonder about the phrase “exercise enforcement discretion”. What precisely does this mean?
If I had to guess, they will choose to go after the clinicians who are using FMT for indications where the evidence for benefit is much weaker (thus far) than for C diff — e$pecially tho$e who $eem to be offering FMT for the wrong rea$on$.
And FYI, there are LOTS of those (still unproven) indications out there, as any brief internet search will reveal — as one site notes, they include “Ulcerative Colitis, Crohns, IBS, Constipation, Autoimmune disorders, Obesity, Parkinsons, Multiple Sclerosis, Anxiety and Depression.”
Will FMT work for some of these? As the cliche (and grant proposals) note, “more research is needed…”
Meanwhile, some were disappointed that the video I linked last time this topic was reviewed was a fake — you wanted the real thing.
Ask and you shall receive.
June 17th, 2013
Gallant is Answering Your HIV Questions and Zuger Writes About the Tough Practice of “Doing Nothing”
Two highly recommended products from a couple of my friends in the HIV/ID world:
First, the inimitable Joel Gallant — long time of Johns Hopkins, soon to be of Santa Fe — has resuscitated his terrific Patient Q & A Forum here. He used to answer patients’ questions regularly on www.hopkins-aids.edu, but that whole site appears to be gone — either truly gone, or Harvard Medical School controls our web searches, nixing anything having to do with a top rival.
(Nope — truly gone. Here’s a little archived material.)
The questions on the new forum are far-ranging in topic — though risk of transmission seems to be a recurring theme — and, not surprisingly with Joel’s writing the answers, the responses are always authoritative, and often very funny.
Fortunately, he’s populated his site with some of his favorites from the Hopkins days:
Is the actual risk of female-to-male transmission greater than the .0001 per contact that I am always hearing? If this is actually the case, it would seem that a policy of promiscuity would actually reduce risk, at least from a male perspective.
I’ve read your question several times now, and each time I read it my jaw drops lower. First, numbers like these are fairly meaningless because they don’t take into account the woman’s viral load, the nature of the sexual contact, the presence or absence of blood or genital ulcers, etc.
But unless I missed something in high school math, any number greater than zero means promiscuity is a bad idea. Engaging more frequently in an activity that has an identifiable risk, however low, cannot reduce the risk of infection. Granted, I took my last math class decades ago. Maybe there have been new developments in the field that I’m not aware of. If you are using an alternative form of mathematics that makes promiscuity not only safe but advisable, please enlighten us! I’m sure there would be great interest!
This one pretty much crystalizes what’s great about his Forum (and, come to think about it, Joel in general) — he can be academic and racy at the same time!
Meanwhile, over at the New York Times, there’s a superb piece by ID/HIV Specialist, Times staff writer, and Journal Watch editor Abbie Zuger.
It’s about how challenging it can be in clinical medicine sometimes to … do … nothing. Citing the example of one of her patents who has persistent and multiple complaints over a decade of care despite innumerable blood tests, scans, treatments, and referrals to specialists, Abbie realizes that what’s best for her is sometimes taking this passive but difficult approach:
That meant not treating her terrible sinus attacks, not investigating her continuing abdominal pain, not medicating her headaches and her crampy hands, not addressing her depression, her itch, her nausea … To really do nothing, all shamanic trappings must be abandoned: stethoscope, prescription pad, weighty pronouncements, the works. And yet — and this is key — doing nothing is also quite different from saying, “There’s nothing I can do for you; goodbye.” Most doctors are masters of this final nothing. But keeping a therapeutic relationship afloat without the usual tools, tricks or enticements — that is a rare achievement, and surely harder than the hardest microsurgery.
I kept nodding my head in recognition during the article; perhaps this is the best line of all: “In fact, one might hazard that doing nothing is the most subversive activity in all of modern medicine, undermining as it does the agendas of all doctors, all patients and all interested corporate parties.”
Maybe it’s no surprise that the reader comments on her piece drew wildly divergent opinions — some wondered how Abbie could bill for her services if she’s “doing nothing”; some accused her of saying that her patient was faking the symptoms (she never said that at all).
Fortunately a significant proportion of commenters realized just how accurate — and important — this practice for “doing nothing” sometimes is in modern medicine. Certainly it’s a whole lot safer than prescribing unnecessary medications or ordering low-yield, expensive, and potentially harmful tests.
Jerry Seinfeld would be proud.
June 13th, 2013
PrEP Works in Injection Drug Users, CDC Offers “Guidance”
From The Lancet comes this important study of tenofovir pre-exposure prophylaxis for injection drug users (IDUs):
In this randomised, double-blind, placebo-controlled trial, we enrolled volunteers from 17 drug-treatment
clinics in Bangkok, Thailand … We randomly assigned [2413] participants to either tenofovir or placebo … 50 became infected during follow-up: 17 in the tenofovir group and 33 in the placebo group, indicating a 48·9% reduction in HIV incidence (95% CI 9·6–72·2; p=0·01).
As with other PrEP studies, better adherence = more protection. There was more nausea and vomiting in the tenofovir treatment group, but no significant differences in serious adverse events. No one who acquired HIV in the TDF arm developed HIV resistance.
So now what? In parallel with publication of the study — which was done in part by CDC investigators — CDC has issued an update on their “Interim Guidance” on PrEP, now focusing on IDUs. Key aspects of the recommendation:
- Consider it only in those at “very high risk” for HIV via IDU, meaning: sharing of injection equipment, injecting at least daily, and use of cocaine or methamphetamine.
- Critical to exclude HIV infection before starting PrEP, monitor regularly for incident HIV, side effects, pregnancy, etc.
- Use tenofovir/emtricitabine (TDF/FTC), even though the Thai study used tenofovir.
The rationale behind this last recommendation, even though the cost of TDF/FTC is higher?
TDF/FTC contains the same dose of TDF (300 mg) proven effective for IDUs, 2) TDF/FTC showed no additional toxicities compared with TDF alone in PrEP trials that have provided both regimens, 3) IDUs also are at risk for sexual HIV acquisition for which TDF/FTC is indicated, and 4) TDF/FTC has an approved label indication for PrEP to prevent sexual HIV acquisition in the United States.
Makes sense to me.
(What also makes sense is that they’re abbreviating it “TDF/FTC”, rather than “FTC-TDF”, as in iPrEx — that was driving me crazy.)
Both the study and the Guidance are welcome additions to the HIV prevention effort. Still, I suspect PrEP in this population will prove particularly difficult to implement, as adherence to medical interventions — for example, HIV treatment — is notoriously challenging among those with active injection drug use.
June 11th, 2013
Both Simeprevir and Sofosbuvir Likely Approved by 2014 — Clinical/Ethical/Pharmacoeconomic Dilemmas Loom
 As expected, simeprevir, and now also sofosbuvir, are being given “priority review” by the FDA.
As expected, simeprevir, and now also sofosbuvir, are being given “priority review” by the FDA.
With the 6-month rule under the Prescription Drug User Fee Act — usually just said as “pah-DOOF-ah” — that means there’s a good chance we’ll have both of these anti-HCV drugs some time in late 2013.
Which also means HCV treaters will soon face a major dilemma regarding management of HCV genotype 1.
For those not following this field closely, here’s the deal:
- If approved, both simeprevir and sofosbuvir will be indicated for treatment of HCV genotype 1, but only in combination with pegylated interferon/ribavirin. (Sofosbuvir approval is also expected for genotype 2/3, just with ribavirin.)
- Both will be one pill once daily. Simple!
- Both will cost a lot. Reminder, a course of telaprevir is $50,000, and these two new drugs are better.
- The interim results of the COSMOS study, presented at CROI this year, showed a >90% cure rate in 80 prior null responders (no cirrhosis) who received simeprevir and sofosbuvir together for 12 or 24 weeks, with and without ribavirin. There was no interferon in this study. (The full slide set of the presentation is on NATAP.)
So if these two drugs are both approved as expected, one could easily make the case that the best treatment for HCV genotype 1 — in terms of efficacy, safety, tolerability, pretty much everything except drug-drug interactions and cost — will be the COSMOS regimen of simeprevir and sofosbuvir, with or without ribavirin. And emphatically without interferon.
And that, my dear friends, is off-label use.
So let’s get inside the head of the various players in this potential drama to imagine what they’ll be thinking.
It’s January, 2014, and a patient previously treated with interferon/ribavirin for 48 weeks who relapsed is coming in to review his new treatment options. Here are some potential thought balloons:
Patient: I just want what works best. Sure would be nice to avoid all those nasty interferon side effects I had the last time I was treated.
Provider: I’d like to prescribe simeprevir plus sofosbuvir to avoid all those nasty interferon side effects the last time he was treated. I have some uncertainty about whether to include ribavirin, and whether to treat for 12 or 24 weeks.
Provider’s RN/PA/NP/PharmD who manages all HCV treatment: Sure will be nice to avoid all those nasty interferon side effects that happened the last time he was treated.
Patient’s insurance company: Both simeprevir and sofosbuvir? Two DAAs? You gotta be kidding me — we’re not paying since the regimen is not FDA-approved. Here’s a toll-free number for you to call and argue the case, but the estimated hold time is longer (by a factor of 10) than trying to call an airline to get a flight changed during a blizzard.
Phamaceutical company: We invested a gazillion dollars to bring these drugs to market. Plus, if you consider the net societal savings by curing HCV and avoiding cirrhosis, liver failure, hepatocellular carcinoma, liver transplants, secondary transmission etc, the price is justified.
Activists: The best treatment should be available for all!
Academic MD expert who has been asked to comment, but isn’t actually seeing this patient (or any patient) today because he/she is writing grants and papers, or traveling: The sample size of the COSMOS study is too small to influence clinical practice.
Of course, this will all settle out once there are multiple non-interferon HCV treatment options out there.
I’m an optimist.