An ongoing dialogue on HIV/AIDS, infectious diseases,
August 24th, 2014
Combination ABC/3TC/DTG Approved — Fourth One Pill a Day HIV Treatment
 As expected, there’s a new single pill regimen for HIV, this one containing abacavir (ABC)/lamivudine (3TC)/dolutegravir (DTG), and it’s called Triumeq.
As expected, there’s a new single pill regimen for HIV, this one containing abacavir (ABC)/lamivudine (3TC)/dolutegravir (DTG), and it’s called Triumeq.
(Where oh where do companies get these names?)
A few thoughts/comments:
- The most important study for this combination was the SINGLE study, which showed that ABC/3TC + DTG (given as two pills) was superior to TDF/FTC/EFV (given as one), the difference based primarily on tolerability advantage of the former. It was a novel double-blind study, as all three drugs were different in each treatment arm.
- Another benefit DTG-based regimens — whether given with ABC/3TC or TDF/FTC as separate pills — is that to date, no treatment-naive patient with virologic failure has developed resistance to DTG. I suspect it will happen one of these days, but the data thus far suggest at the very least DTG resistance will be a rare event.
- Needless to say, but will say it anyway for emphasis, all patients starting this regimen will need to be tested for HLA-B*5701 and found to be negative. Wonder if pharmacies will enforce this, or whether it will be left up to the prescribers. Here’s the landmark study that proved this testing essentially excludes severe hypersensitivity to ABC, quite an amazing story in pharmacogenomics.
- An unresolved question is whether ABC is associated with an increased risk of cardiovascular disease. This FDA meta analysis of randomized clinical trials didn’t think so, but around half of the observational studies did, including the updated DAD study. NA-ACCORD data will be of great interest, whenever they appear.
- As of Sunday, August 24, the price of ABC/3TC/DTG is not known (at least to me) — and it could be a real game changer, since both ABC and 3TC are already generic. Says Ben Young over on TheBody: “If ViiV Healthcare manages to set the price of Triumeq in accordance with the generic status of abacavir and lamivudine, the cost should be substantially lower than the price of the other single-tablet regimens that contain all on-patent components.” Yep.
- I suspect people will call it “Trii” (rhymes with “Cy”, as in “Cy Young”) for a while, just like they called TDF/FTC/EVG/COBI “Quad”.
So what are some upcoming single-tablet regimens? Tenofovir alafenamide (TAF) with FTC/EVG/COBI. TAF/FTC/DRV/COBI. DTG/rilpivirine. And inevitably, generic TDF/3TC/EFV, which is widely available globally.
Regardless, sure beats the old days.
August 16th, 2014
Dietary Advice From Your Friendly ID Doc: Don’t Eat Garden Slugs
 From the pages of Open Forum Infectious Diseases, comes this cautionary case report:
From the pages of Open Forum Infectious Diseases, comes this cautionary case report:
Toxocariasis After Slug Ingestion Characterized by Severe Neurologic, Ocular, and Pulmonary Involvement
I encourage you to read the full paper, but the short story is that a previously healthy 71-year-old man was admitted to a hospital in France with fever, cough, headaches, confusion, and eosinophilia. A comprehensive (that’s an understatement) work-up found elevated antibody levels to Toxocara canis, otherwise known as the dog roundworm.
But the case truly hinges on this sensational sentence.
One month later, a reassessment of the case history revealed a long-standing daily intake of 2 or 3 raw local slugs for alternative therapy of gastroesophageal reflux; this information prompted us to perform further investigations.
Vive La France!
And oh yeah, ID doctors take the best medical histories.
August 10th, 2014
Waiting (and Preparing) for Ebola
 For Infectious Diseases doctors, there’s a certain life cycle to the big ID topics that make their way to the lay press, and it’s playing out right now big time with the terrible Ebola outbreak in Western Africa. It goes like this:
For Infectious Diseases doctors, there’s a certain life cycle to the big ID topics that make their way to the lay press, and it’s playing out right now big time with the terrible Ebola outbreak in Western Africa. It goes like this:
- Someone reports an outbreak in a venue like ProMED.
- Almost synchronously, it is covered by the news.
- Depending on the severity of the outbreak and the Gladwellian “stickiness factor,” it then becomes well known outside of the ID community. And boy, does Ebola have plenty of stickiness — much more than chikungunya or listeria in fruit, for example, to cite some recent entities that grabbed a fair amount of attention.
- Suddenly everyone wants to talk to you, Dr. ID Person, about it — the orthopedic surgeon (two of them mentioned Ebola to me last week!), the ICU nurse, the people you play canasta with (I don’t play canasta, just assuming), your Aunt Becky (I do have an Aunt Becky), and even complete strangers who, in the course of serving you food at a restaurant or fixing your car or changing the grip on your badminton racket, find out that you’re an ID doctor. You’re the expert, after all.
The problem is that going from Item #1 to Item #4 can happen awfully fast. And because these newsworthy outbreaks frequently haven’t played out before — otherwise they’d be less newsworthy — it’s virtually guaranteed you are not in fact an expert. At least not yet.
Which sends you (and all of us, trust me) scurrying to the great big web in the sky for some intense reading on the disease du jour.
So for the ID doctors reading this out there, please share your best sources for the latest in Ebola news and information. CDC, of course, and the aforementioned ProMED, the prescient HealthMap and if you want to drink from a fire hose of lay coverage, the Google news feed — where else?
And for non-ID readers, cut your ID docs a little slack if we don’t instantly know the answer to your Ebola questions. We’re working on it!
In the meantime, please don’t eat plums eaten by bats.
July 31st, 2014
Simeprevir, Sofosbuvir, and the Limitations of the COSMOS
 These are exciting times for hepatitis C treatment, as the approval of simeprevir and sofosbuvir in late 2013 have made curing this disease a whole lot easier.
These are exciting times for hepatitis C treatment, as the approval of simeprevir and sofosbuvir in late 2013 have made curing this disease a whole lot easier.
Since that sentence barely conveys the transformative nature of this medical advance, allow me this tortured analogy: Before simeprevir and sofosbuvir, curing hepatitis C was like making a transatlantic journey by ship, and you had to stay in steerage — always a long and painful trip, but you’d get there if you could stand it. Now the cure is like a business-class flight — much shorter, safer, and more comfortable, but you just have to have the resources to pay for it.
But our treatments are not perfect. I was reminded of this fact when someone treated with simeprevir and sofosbuvir — or “SIM-SOF” as it’s commonly abbreviated — just relapsed a month after finishing 12 glorious, side-effect–free weeks of treatment.
In hindsight you could perhaps have predicted that this patient — or this type of patient — would be a treatment failure on these new drugs. There was prior treatment failure on interferon/ribavirin; he has genotype 1a (and hence probably Q80K, didn’t test for it); he’s overweight; he has several other medical problems, including diabetes.
And, probably of greatest importance, he has cirrhosis, diagnosed by liver biopsy several years ago.
The treatment failure sent me scurrying over to the reason we use this combination anyway — the excellent HCV treatment guidelines and the remarkable COSMOS study (just published in the Lancet) of simeprevir and sofosbuvir, with and without ribavirin, in patients with HCV genotype 1.
Here’s what recommended in the guidelines for patients who have failed prior treatment:
Daily sofosbuvir (400 mg) plus simeprevir (150 mg), with or without weight-based RBV (1000 mg [75 kg]) for 12 weeks is recommended for retreatment of HCV genotype 1 infection, regardless of subtype or IFN eligibility.
And here are the relevant data cited:
Among those null responders with a Metavir fibrosis stage of 3 or 4 (n=47) who received 12 weeks of sofosbuvir and simeprevir, SVR4 was observed in 14 (93%) of 15 patients in the ribavirin-containing arm and 100% (all 7 participants) in the RBV-free arm.
A few things stand out from reviewing the study and the guidelines, aside from the high response rate:
- The sample size in the COSMOS study was small.
- The number with actual cirrhosis was even smaller, as they combined stages Metavir 3 and 4.
- The number with actual cirrhosis who did not receive ribavirin and received only 12 weeks of treatment was smaller still.
- And did I mention — the sample size in this study was small?
The reason I’m harping on the sample size is a lesson taught early in every Stats 101 course — namely, that a small sample size means that the outcome will not be very precise, with lots of potential wobble around the results.
The most recent data presentation of the COSMOS study allows us to drill down a bit more on the data. There were 7 patients — just 7 — with cirrhosis who received only simeprevir and sofosbuvir for 12 weeks. 6 out 7 were cured (SVR 12), for a response rate of 86%.
And the lower bound of the 95% confidence interval for this proportion? 60%, at least according to this web gizmo that does the math for us, if I’ve chosen the right “equation”. So the occurrence of relapses should not be a huge surprise, despite the > 90% response rate for the study overall.
Some take-home lessons:
- Unlike the real thing, the COSMOS study is very small. (There’s a larger study of this combination ongoing.)
- This small sample size means we can’t predict response rates with much accuracy, especially in the hardest to treat patients who were not heavily represented in the study.
- SIM-SOF (note it’s rarely “SOF-SIM”) is still a great advance in HCV treatment, but treatment failures will happen.
So what to do next? The good news is that resistance to sofosbuvir is rare even in treatment failures, and that the HCV treatment cosmos is about to get bigger — the next class of drugs (NS5A inhibitors) is on the way soon, and the data on sofosbuvir plus ledipasvir or daclatasvir look very promising.
A reason for optimism even for those for whom SIM-SOF didn’t do the trick.
July 30th, 2014
Hepatitis Day “Celebration” and a Reminder
July 28 is “World Hepatitis Day” (how do they choose the dates for these things?), and I wrote a bit over on the Oxford University Press site on the incredible progress we’ve made already — with more to come.
Definitely plenty of reasons to celebrate — safe and effective immunizations for hepatitis A and B, treatment (non-curative) for hepatitis B, and astounding therapies now for HCV. Still, given the global magnitude of these infections, and the problems of access, there is plenty of work to be done to get people immunized, diagnosed, referred, and treated.
Enjoy the tune.
July 27th, 2014
Really Rapid Review — AIDS 2014, Melbourne
![]() For the second time — the first time was in Sydney, 2007 — the annual “summer” international AIDS conference took place in Australia, this time in Melbourne way down in the southern part of the country. I’ll note again how the crash of Malaysia Airlines flight MH-17 cast a sad note over the opening sessions, and throughout the conference many people gave moving tributes to friend and colleague Joep Lange.
For the second time — the first time was in Sydney, 2007 — the annual “summer” international AIDS conference took place in Australia, this time in Melbourne way down in the southern part of the country. I’ll note again how the crash of Malaysia Airlines flight MH-17 cast a sad note over the opening sessions, and throughout the conference many people gave moving tributes to friend and colleague Joep Lange.
This being the larger of the two international meetings, there was plenty of non-medical, non-scientific content, all of which I’ll completely pass over in this Really Rapid Review©.
(Except to say that Bill Clinton and security entourage walked right past me — he waved hello, though clearly not to me personally.)
With the usual up-front apologies for inadvertently omitting something important, off we go.
- When given with darunavir/ritonavir, maraviroc was inferior to TDF/FTC for initial therapy. We knew this already from the press release last year, but this was the first time we’ve seen the data. Failure rates particularly high with lower CD4 cell counts. Interesting conference dynamic when the presenting author was asked about whether the dose of maraviroc (150 mg mg daily) was too low — he responded rapid-fire with around 5 studies justifying the dose, quite a performance.
- As maintenance therapy, atazanavir/ritonavir plus lamivudine (two drugs) was non-inferior to atazanavir/ritonavir plus 2 NRTIs (three drugs). As with the GARDEL study of LPV/r + 3TC, these results are still more evidence of the magic of 3TC/FTC. Study is called “SALT” — can you guess what this stands for?
- As maintenance therapy, lopinavir/ritonavir plus lamivudine (two drugs) was non-inferior to lopinavir/ritonavir plus 2 NRTIs (three drugs). Again … magic of 3TC/FTC! And this one was called “OLE” — another clever title, please guess the origin.
- Given the results of the above three studies, perhaps we shouldn’t be surprised that for virologically suppressed patients, raltegravir plus ATV/r was clearly worse than ATV/r plus 2 NRTIs — the study was stopped by the DSMB for more virologic failures in the experimental arm. What’s causing the underperformance of these two drug regimens that don’t have 3TC/FTC? To quote my friend Joel Gallant, “the only good nuke-sparing regimens contain a nuke.” (When you steal a line that good, you must give credit.)
- The SECOND LINE study found after failing 2NRTIs/NNRTI, second-line treatment with lopinavir/r with either raltegravir (two new drugs) or NRTIs (one new drug plus recycled but still partially active drugs) were non-inferior. In this resistance analysis of the study, having more resistance at study entry lead to a higher likelihood of treatment success. A paradox? Not really, this has been seen before — patients with the worst adherence have the least resistance, hence they do poorly on their next regimens too.
- The SAILING study showed that dolutegravir was superior to raltegravir in treatment experienced patients, and this detailed resistance analysis found that the difference was quite pronounced in those treated only with NRTIs. 0/32 receiving DTG plus NRTIs failed treatment, vs 7/32 for RAL plus NRTIs; for M184V +/- thymidine-associated mutations, there 0/13 DTG vs 4/12 RAL failures.
- In this large analysis of a Spanish treatment cohort, HIV RNA between 20-50 cop/mL did not increase the risk of treatment failure compared to those with HIV RNA < 20 cop/mL. Reassuring, because we don’t know how to manage those patients anyway!
- In an observational Canadian cohort study, patients who switched therapy while virologically suppressed had significantly greater risk of failure than those who didn’t switch. Not surprisingly, the switchers vs non-switchers differed substantially — enough so that this study was this meeting’s winner of the “Unmeasured Confounding Influenced Outcome” award, a limitation the presenter acknowledged.
- How often should we be measuring viral loads in our stable suppressed patients? In this HIV Outpatient Study (HOPS) analysis, virologic suppression rates were similar between those who did and did not have HIV RNA measured more than twice a year. Could save a boatload of money if the frequency of these tests could be reduced in selected stable patients.
- What are some of the predictors of prescribing guidelines-concordant HIV treatment in the USA? Glad to see that being an ID specialist was one of them! (Love the title of the abstract too…)
- Tons on PrEP at the meeting, but the study that got the most attention was an interim analysis of a French study of intermittent PrEP, called “IPERGAY”, which stands for … something. Adherence excellent so far in this “event driven” strategy, no outcomes data yet. Note that the comparator arm to the intermittent PrEP is placebo, as PrEP is not approved or strongly endorsed in France.
- More indirect evidence that intermittent PrEP could be the way to go from this iPrEx analysis, which found 100% protection in those participants whose blood levels suggested 4X/week adherence to daily TDF/FTC.
- Don’t share cuticle scissors with someone who is viremic. Enough said.
- There’s no doubt that HIV positive MSM have more anal dysplasia and anal cancer than their HIV negative counterparts, and high grade squamous intraepithelial lesions (HSIL) are thought to be a strong precursor to cancer. But this well done Australian study found that more than half of HSIL lesions regressed spontaneously, making the optimal treatment uncertain. Great name for the study, by the way — Study of the Prevention of Anal Cancer (SPANC).
- In HIV/HCV coinfection (genotypes 1-4), sofosbuvir plus ribavirin cured over 80% of patients, with perhaps the only weakness the genotype 1a patients with cirrhosis. This combination remains the treatment of choice for genotype 2; for genotype 1, sofosbuvir/ledipasvir is imminent.
- Speaking of imminent approvals for genotype 1 HCV, the “3D” regimen of ABT-450/r/ombitasvir (three drugs coformulated) plus dasabuvir plus RBV cured over 95% of coinfected patients. Interestingly, some of them received atazanavir-based regimens, using the “r” (ritonavir) in the HCV regimen to provide the boost.
- A small study of this same combination found that it was safe and effective (again, cure > 95%) in patients on methadone or buprenorphine, with no adjustments required of the narcotic replacement therapy.
- Cure research has taken a beating recently, especially with the virologic relapses of the two Boston stem cell transplant patients and the Mississippi baby. But the research goes on, and here a study demonstrated clearly measurable increases in HIV RNA after infusions of the potent HDAC inhibitor romidepsin — suggesting a reversal of HIV latency! The current thought is that this sort of treatment plus other measures (vaccine? immune augmentation otherwise?) may decrease the latent reservoir.
A few brief impressions of Melbourne, top of the World’s Most Livable City rankings by The Economist since 2011.
- The people were uniformly nice, polite, energetic, and helpful. All of them! There must be some ornery and disagreeable Melbourne natives someplace, but they are either extremely small in number or they are carefully hidden away, far from where tourists roam.
- As a tennis-crazy person, even though I knew it was illogical, I kind of expected Melbourne to be hot — think of the Australian Open in January, matches held in blistering 40 degree celsius heat with blinding sun, players taken into the locker room for IV hydration, etc. Of course this city’s location in the way south of Australia means it’s not “summer” here at all (see the first line up there), it’s just flat-out winter — cool, cloudy, kind of like Seattle in October/November — but nothing (and I mean nothing) like a Boston winter. Wear a sweater and/or a light coat and you’re fine.
- The city has the largest street tram service in the world, and somehow it all works: the streetcars don’t seem to interfere with the traffic or pedestrians, and the stops have incredibly clear and helpful signs telling you not only how long you need to wait for the next car, but the next 3 cars after that. Imagine that on the Boston T on Commonwealth Avenue!
- Food is outstanding, with strong Asian influences everywhere, and many of the restaurants are charmingly located on narrow alleyways, with indoor/outdoor dining overlapping to make the alleys seem like outdoor rooms. Tipping is barely required — kind of viewed as sort of a strange American custom. I asked about tipping in one restaurant, as I didn’t have the right currency for both the bill and a tip, and the waitress said (in all sincerity), “That’s very kind of you, but we’re just fine, thank you.”
- This is a city of true coffee fanatics, proven by the fact that there are only two Starbucks in all of Melbourne — but don’t go to them! From humble little snack bars to high-end bean emporia, the quality of the coffee here is off the charts sensational. Maybe that’s why everyone is so nice? Helpful for jet lag adjustment too.
- Given all of the above wonderfulness, perhaps it’s no surprise that Melbourne is no bargain. (#6 most expensive city in the world, according to this list.) Not eager to see my next credit card statement, yikes.
Next year’s meeting is in Vancouver, which no doubt will celebrate the incredible 1996 Vancouver meeting that introduced effective HIV therapy to the world. Or, to quote Kevin DeCock:
The International AIDS Conference in Vancouver is synonymous with the introduction of highly active antiretroviral therapy (HAART), now just referred to as ART.
He said it, not me!
July 19th, 2014
Mood Solemn in Melbourne as AIDS 2014 Starts
There is unquestionably a shadow cast over this year’s international AIDS meeting here in Melbourne, and it’s not the result of the Australian winter. It’s the crash of Malaysia Airlines Flight 17, of course, which prematurely ended the lives of hundreds of people — including many people en route to this conference, most notably one of the field’s finest clinical researchers and leaders, Joep Lange.
I of course knew Joep (much better than he knew me), and greatly respected his work. He is especially well known for his efforts to make HIV treatments available globally. This achievement is perfectly summarized by Marty Hirsch, quoted in today’s Boston Globe:
He was a leading light, and he was a person who pushed probably as hard or harder than anyone for equity in the field, to make the drugs that we were using in the US and Netherlands available at a low cost for people in underserved areas of the world.
One of his research collaborations is particularly noteworthy given the location of this year’s conference: Along with David Cooper from Sydney and Praphan Phanuphak from Bangkok, Lange was one of the founders of the HIV-NAT Research Collaboration, with NAT standing for “Netherlands Australia Thailand”. This productive effort started in 1995 — one year before combination ART became standard of care! — and was a true model of how countries with diverse backgrounds and economic resources could work together to improve HIV care and research.
Needless to say, everyone here is heartsick about this senseless loss.
July 14th, 2014
Despite Baby’s Relapse, HIV Cure Research Marches On
The big news in the HIV world over the past week was of course the virologic rebound of the Mississippi Baby, who up to this point was considered possibly the second person cured of HIV.
(Timothy Brown — stem-cell transplant recipient from CCR5 negative donor — remains the first and only HIV cure. Every report on this topic needs to mention this, so now I’ve checked that box.)
Obviously this baby’s relapse after over 3 years off treatment was tremendously disappointing — a “punch in the gut” according to one researcher — and, along with the relapse in the Boston stem-cell transplant cases, underscores just how difficult it will be to find a viable strategy for curing this infection.
But it’s equally important to emphasize that significant clinical research in this area has by no means stopped. There’s a vigorous research agenda ongoing in HIV cure, as demonstrated by several studies ongoing in the research network in which I participate (there’s my disclosure), the AIDS Clinical Trials Group. Here are three approaches:
- ACTG A5135: Romidepsin is an in vitro potent HDAC inhibitor (you knew that, right?), which potentially can stimulate latent HIV and hence make it more vulnerable to eradication. You’ll hear more about romidepsin in the upcoming AIDS 2014 Conference in Melbourne, and this ACTG study is enrolling now.
- ACTG A5326: The anti-PD-L1 antibody BMS-936559 can reinvigorate a “fatigued” immune response to HIV, which, along with other strategies, could lead to clearance of the virus. Another study enrolling now.
- ACTG A5308: HIV controllers — those who have undetectable or nearly undetectable HIV RNA even without ART — have smaller HIV reservoirs than most people with HIV. This study will evaluate whether standard ART can reduce this reservoir further, along with improve immunologic abnormalities sometimes observed in these patients. Yes, still enrolling interested participants.
Now none of these will actually cure HIV on their own, but they’re potentially promising first steps. Think of the participants in these studies like these Mercury astronauts, to borrow an analogy from my friend and colleague Joe Eron.
And of course this is just a partial list, with many studies outside the ACTG network also ongoing or planned — including panobinostat (another HDAC inhibitor), zinc finger nucleases, the anti-env cytotoxin 3B3-PE38, other novel gene therapy approaches … Who knows what we’ll hear about next week in Melbourne?
And after that, we can expect more, as there is a tremendous motivation to find the analogous cure strategies to what we have now for HCV. It might be more difficult, but think about the technologies available to us now that barely existed when HIV first was discovered. Kind of like comparing the first home PC to what we have now on our smart phones.
So if the Mississippi baby isn’t cured, and neither are the Boston patients, that doesn’t mean it won’t happen eventually.
Here’s betting it will. A lot of very smart people are working on it, and that’s by no means a comprehensive list. People with lots of brains, unlike the scarecrow.
Enjoy the clip.
June 28th, 2014
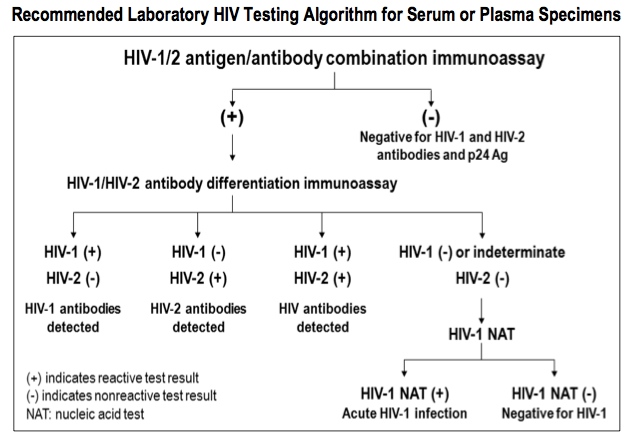
CDC Nixes HIV Western Blot in Latest Testing Guidelines
Finally, it’s official — the Western blot is no longer recommended as a confirmatory test for HIV infection. From the latest Laboratory Testing for the Diagnosis of HIV Infection, updated June 27:
The HIV-1 Western blot and HIV-1 immunofluorescence assay, previously recommended to make a laboratory diagnosis of HIV-1 infection, are no longer part of the recommended algorithm.
It’s been clear now for years that the Western blot’s sensitivity for early HIV infection was markedly inferior to the latest generations of HIV screening tests; this difference became even starker with the approval of the combined “fourth generation” antigen/antibody tests.
This put clinicians in the bizarre situation of having to ignore, or disregard, the test that’s supposed to be more accurate than the screening test. Essentially every patient with a positive ELISA and negative Western blot would need a follow-up HIV RNA test of some sort to exclude early HIV. Read here for more information.
I’ve pasted the new recommended algorithm below. Let’s hope that labs that still use the old “reactive ELISA –> Western blot confirmation” practice quickly move to this new approach. And kudos again to HIV Testing Guru (yes, that’s his official title) Bernie Branson for getting this important change into the guidelines.
June 24th, 2014
At Long Last, Some New Antibiotics That Might Make a Difference
 Compared to HIV, HCV, and antifungal agents, development of novel antibacterial drugs has been in a bit of a funk for quite some time now.
Compared to HIV, HCV, and antifungal agents, development of novel antibacterial drugs has been in a bit of a funk for quite some time now.
Here’s what’s been approved in the past 10 years, with year of approval and a few comments:
- Telithromycin (2004): The first ketolide antibiotic, it was supposed to overcome macrolide-resistant respiratory pathogens. But there were huge controversies about the clinical trials that led to its approval, and major concerns about liver and other adverse effects. It’s still available, but no one in their right mind would use it.
- Tigecycline (2005): Although this incredibly broad-spectrum tetracycline-like agent seemed like just the ticket based on its in vitro activity, clinical experience has been only fair at best. It achieves low blood levels, so isn’t suitable for bacteremia, and is associated with extremely high rates of nausea. Plus there’s this excess death issue in pooled data from several clinical trials — never a good thing!
- Doripenem (2007): The most recently approved carbapenem, doripenem was shown to be inferior to imipenem for ventilator-associated pneumonia, with a lower rate of cure and a higher risk of death. (Bad.) That trial alone — sponsored by the makers of the drug, no less — should be sufficient reason never to prescribe it.
- Telavancin (2009): A relative of vancomycin, telavancin is approved for skin infections. It appears to be more nephrotoxic than vancomycin, however, and offers few advantages. I still have never prescribed it, and our ID PharmD says there has been only one patient in our hospital’s history who has received it.
- Ceftaroline (2010): The first cephalosporin with anti-MRSA activity, ceftaroline has a relatively narrow list of FDA-approved indications — namely, community-acquired pneumonia (who would use it for this?) and skin and soft tissue infections. As a result, the drug is really still trying to find itself, if I may personify Mr. C. Fosamil for a moment. What we’d really like to know is how it does for MRSA bacteremia and other severe infections, but thus far the data for these indications are quite limited.
- Fidaxomicin (2011): This novel treatment for C. diff may be slightly more effective than vancomycin, at least in certain populations. It is extremely expensive — it’s the current winner in the “Most Expensive Oral Antibiotic” competition — so anecdotally it’s almost always used as a second-line or salvage therapy. In addition, FMT and other microbiome-restoring treatments may ultimately make use of antibiotics for C. diff of limited use.
I’ve probably left some antibacterial out from the above list (did it by memory, except for the FDA approval dates — always risky), but think this pretty much covers the decade. Aside from ceftaroline — which again, is still kind of in a transitional phase as we gather more data — it’s not a particularly impressive group of drugs.
Hence the recent approvals of dalbavancin and tedizolid, and the likely upcoming approvals of oritavancin, ceftazidime/avibactam and ceftolozane/tazobactam, all give ample reason for hope. These incentives seem to be working!
Dalbavancin and oritavancin, with their prolonged half-lives and infrequent administration, could “profoundly affect how skin and soft tissue infections are managed, by reducing or in some cases eliminating costs and risks of hospitalization,” to quote this nice editorial in the New England Journal of Medicine.
Tedizolid is (finally) an alternative to linezolid, with once-daily dosing and likely a better safety profile.
And the two new cephalosporin/beta-lactamase inhibitor combos could provide much-needed options for highly resistant gram negative infections, which we hope would obviate or at least reduce the use of tigecycline and colistin.
Sure, we still need to see these drugs in the “real world” before we know about effectiveness, tolerability, safety, and price. But I’m cautiously optimistic.