An ongoing dialogue on HIV/AIDS, infectious diseases,
July 25th, 2024
Lenacapavir PrEP Trial Brings Down the House at the International AIDS Conference
Yesterday, at the 2024 AIDS Conference in Munich, we experienced one of those thrilling moments you always hope for when attending a scientific conference.
Dr. Linda-Gail Bekker, speaking on behalf of the study investigators, presented the data on the PURPOSE-1 study of HIV prevention using twice-yearly injectable lenacapavir; results were simultaneously published in the New England Journal of Medicine.
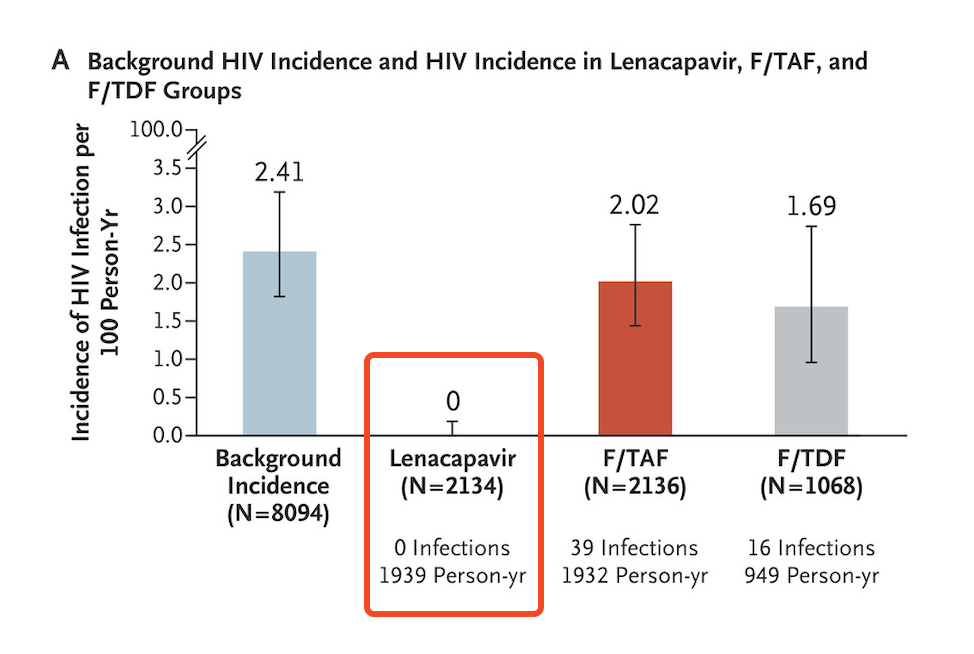
The results originally made headlines when the study was halted by the external independent data monitoring committee in June — some examples:
Twice-yearly Injection Fully Protects Women from HIV
New Drug Provides Total Protection From HIV in Trial of Young African Women
Lencapavir Shows 100% Efficacy and Zero Infections in HIV Prevention
Beginning of End of HIV Epidemic?
Certainly, all of us at the conference knew the results before yesterday’s presentation of the data. Regardless, when the results slide flashed on the screen, highlighting zero infections in the lenacapavir arm, there was spontaneous applause from the thousands assembled in the cavernous convention center:
How could we help ourselves? Several attendees today reported to me they had tears of joy. At the end of the presentation, there was a standing ovation — one given not just to Linda-Gail for her excellent summary, but also to the other study investigators, the participants, the sponsor, and the scientists who have made so much progress in HIV prevention that we can only rejoice.
There’s a lot to commend about this clinical trial, it’s hard to know where to begin, but here’s a partial list:
- The investigators carefully engaged with the community at risk while designing the study.
- It was conducted in sites known to have a high incidence of HIV in young women, then enrolled those at highest risk (over 20% had an active STI at enrollment).
- They used a novel approach to estimating background incidence, relying on those who failed screening due to already having HIV, then applying “recency” assays to estimate the timing of when these infections occurred. It’s a complex calculation, but it avoids having a placebo arm, which of course would be unethical.
- The study was blinded, strengthening the conclusions from the data.
- The primary and secondary end points were of great interest. Note that in addition to lenacapavir being superior to both no PrEP (background incidence) and TDF/FTC, the study also showed that TAF/FTC and TDF/FTC were not meaningfully different and that TAF/FTC only provided protection with good adherence — which was unfortunately not common among those assigned to oral therapy. (The efficacy of TDF/FTC was not a defined endpoint.)
- Participants who became pregnant or were breast-feeding could continue the study with outcomes incorporated into the protocol. Such end points are critical for this study population, but too rarely assessed.
A second study in a different at-risk group (predominantly MSM) is ongoing, PURPOSE-2, with results expected within the next 6 months or so. If it has comparably favorable results, at that point the company can file for regulatory approval, which presumably will be swift.
Then, as noted in the accompanying editorial in NEJM, other challenges loom — implementation, access and cost. These are challenges everywhere, not just in Africa where the study was conducted.
Indeed, we know how difficult injectable PrEP is to start in our clinics, even though cabotegravir gained FDA approval for this indication by being superior to oral TDF/FTC in two excellent clinical trials (HPTN 083 and 084). In fact, the 084 study of injectable cabotegravir given every two months demonstrated a similar 100% protection from HIV among the women adherent to the injection schedule.
Despite these favorable results, and patient interest in injectable PrEP, someone told me that less than 5% of PrEP in the United States is now with cabotegravir. The primary reasons for underutilization relate to strained clinic resources (someone has to give the injections), cost, drug access, and manufacturing.
Still — zero infections PURPOSE-1. Wow. If you are running a clinical trial, you dream about getting results like this.
Let’s join the applause.


Wonderful. Reminds me of when we first heard about the confirmatory “U=U” studies. Also applause.
Lenacapavir gave 100% protection from HIV in PURPOSE-1 study. However, Gilead is currently charging over $40,000 per year for this drug in the US, France, Spain, Norway and Australia. We have calculated that lenacapavir could be mass produced as a generic for under $100 per person-year. We will need to achieve these low prices for lenacapavir to be used in mass prevention campaigns, protecting those at highest risk from HIV infection. Millions of people will need to be treated to lower worldwide rates of HIV infection. There are 1.3 million HIV infections each year – one every 24 seconds.
Gilead need to sign voluntary licenses with the major generic manufacturers as soon as possible, so that mass production can be started. It does not make sense to wait for the results from other randomised trials before making this decision. Over 50% of global HIV infections are in women, so the results from PURPOSE-1 should provide enough evidence to start mass generic production now. A voluntary license covering all low- and middle-income countries would allow the use of lenacapavir in 83% of the world’s population, where 95% of HIV infections are seen.
An amazing advance.
Agree that it all will be wasted if not made available in the regions that need it the most.
It’s been so hard to coordinate injectable cabotegravir PrEP in our healthcare system (a small network of community-based primary care clinics). Insurance, purchasing, scheduling injections, etc. Much harder than just starting someone on Truvada, which is now generic and cheap.
Wondering (hoping!) that this will be better since it’s only 2x/year instead of 6.
This drug is definitely a game changer for the HIV arena ( Naive, HD Experienced, PreP) and we
realize that Pharma needs a fair share profits of it`s investment, BUT.. If we really what to curtail
and end the HIV epidemic, the moment is now.
It needs to be discussed FAST among the policies and politics of government`s and positioned as a high priority making it available to the world.
Wonderful news for clinicians in the care of PWH. The results are just awesome!!
This makes us consider how actionable these results are, considering the availability in LMIC, cost, and other STIs looming.
Looking forward.
Thanks
Wonderful. Reminds me of when we first heard about the confirmatory
Visit Us Telkom University Jakarta