An ongoing dialogue on HIV/AIDS, infectious diseases,
December 20th, 2021
Believe It or Not, We Already Have a Highly Effective Outpatient Antiviral Treatment for COVID-19

A pine tree. With a colorful birdhouse.
And that treatment is … (drum roll) … remdesivir.
Yes, you heard me right. Remdesivir, the very same antiviral with a checkered history in COVID-19 clinical trials, with some studies showing efficacy (sort of), others not much of anything.
What’s the truth here?
For that, let’s turn to what gets my vote for the most unheralded highly favorable clinical trial result since the start of the pandemic, the PINETREE study.
The trial randomized 562 outpatients with an increased risk for severe COVID-19 to daily intravenous remdesivir for 3 days or placebo. None had vaccination or treatment with monoclonal antibodies.
The results were unequivocally positive — remdesivir-treated patients had an 87% reduction in the risk of hospitalization or death, with no significant treatment-related toxicities. Key figure from the IDWeek 2021 late breaker presentation:
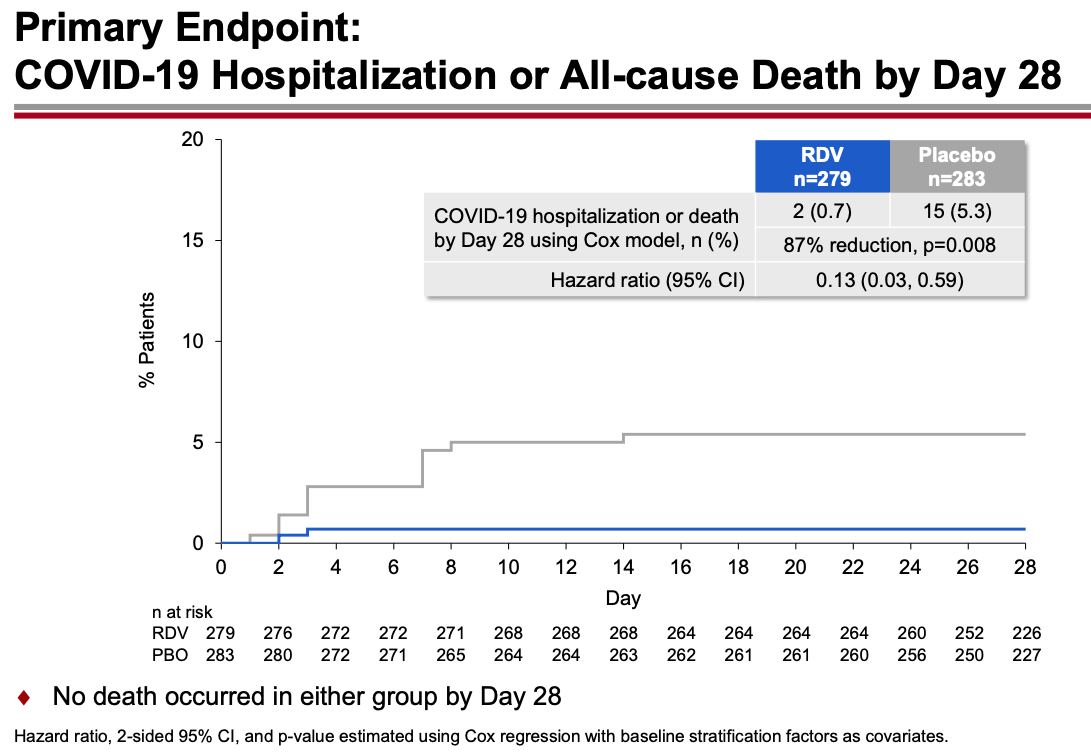
The contrast with the so-so results of the drug in hospitalized patients is striking, and underscores the importance of early antiviral treatment for this disease. By the time the immune-mediated organ damage kicks in — which is when most hospitalizations take place — it’s often too late for antivirals to have any effect.
Note that remdesivir given early in the course of illness to outpatients yields comparable results to the monoclonal antibodies and nirmatrelvir plus ritonavir (Paxlovid), and better results than molnupiravir. (Yes, caveats about cross-study comparisons understood.) Furthermore, remdesivir has been available for months, and is the only fully FDA-approved treatment for COVID-19 — which means clinicians can prescribe it in whatever setting they deem useful.
But it’s hard — OK, nearly impossible — to find anyone who has actually used remdesivir in a nonhospitalized patient:
The PINETREE study showed that remdesivir daily for 3 days in nonhospitalized, high-risk individual with Covid19 reduced the risk of hospitalization by 87%.
Have you or anyone you know used it this way outside of a research study? https://t.co/o9e0IBPH0F— Paul Sax (@PaulSaxMD) December 19, 2021
The reasons so few have adopted this treatment are obvious. Setting up outpatient intravenous therapy urgently is a challenge even under the best of circumstances — and doing so for patients who are acutely ill and have a highly contagious infectious disease only makes it more challenging.
The study sponsors recognized these hurdles and stopped the study early because of the availability of single-infusion monoclonal treatment and vaccine availability, both of which seemed destined to make the study results of limited clinical utility.
But with Omicron’s arrival, the results of PINETREE instantly become quite relevant. Omicron is so highly contagious and vaccine evasive that many people who have been “fully vaccinated” (time to retire that phrase, by the way) will contract COVID-19. My (very funny) British ID colleague Neil Stone describes it as follows:
Forget airborne, this thing seems to spread by telepathy — feels like you can catch it just by THINKING about it.
Furthermore, the most widely used monoclonal antibodies in current use — casirivimab and imdevimab and bamlanivimab and etesevimab — don’t neutralize Omicron. Sotrovimab, which is predicted to retain some activity, is in very short supply. The oral antivirals nirmatrelvir and molnupiravir are not yet available, and even once they get approved, supplies will be severely limited. Note again that molnupiravir reported lower efficacy than remdesivir, and furthermore has mutagenesis concerns.
My late colleague Francisco Marty — a brilliant clinical researcher — proposed studying remdesivir as a single IV dose, analogous to the one-dose baloxavir and peramivir treatments for influenza. This would greatly facilitate outpatient and emergency room treatment. Remdesivir infusions don’t take long, and require no post-treatment observation. Unfortunately, the study never went forward.
Maybe it’s time to revive the idea? Start a clinical trial now comparing 1 vs. 3 doses, using a noninferiority design?
Regardless, we’re going see a lot of COVID-19 in the next few weeks. Since most PCR assays do not report “spike gene target failure” — a hallmark of Omicron — clinicians will not know in real time whether these cases are Omicron or Delta, and hence whether the currently available monoclonal antibodies will work.
But based on other regions where Omicron appeared, the dominance of this variant is all but inevitable and will occur rapidly. This experience in Houston is typical, one we’re seeing in multiple areas in the United States right now:
Sunday Update: The Omicron variant is now in Houston in full force, accounting for 82% of new symptomatic Houston Methodist #COVID-19 cases as of earlier this week.
#Omicron became the cause of the supermajority of new Houston Methodist cases in less than three weeks. pic.twitter.com/XL5C31P4As— S. Wesley Long (@drswlong) December 19, 2021
Why not prepare for this change by shifting the resources we’ve developed for intravenous monoclonal antibodies to intravenous remdesivir? Let’s offer this first to our highest risk patients — people who are B-cell depleted, transplant recipients, other severely immunocompromised.
It won’t be easy, but it will be worth it. Let’s use all the effective tools we have to treat this disease, and that includes remdesivir for high-risk outpatients.


Just heard about the PINETREE study at Grand Rounds on Friday. The speaker intimated that outpatient Remdesevir would not get off the ground for the logistical reasons you noted above.
Would you use a single, standrad dose? Seems like the intracellular half-life is long enough(?).
Interesting, but an IV medication can’t easily be scaled up given America’s weak health infrastructure.
We need a single payer/ Medicare for all and a real focus on prevention.
Here is a link to the study…
https://www.gilead.com/news-and-press/press-room/press-releases/2021/9/veklury-remdesivir-significantly-reduced-risk-of-hospitalization-in-highrisk-patients-with-covid19
It seems reasonable to shift existing units set up for outpatient MAB therapy for COVID patients to remdesivir, but the more practical study would be to study it in the ED where giving a single dose is feasible in patients who don’t merit hospitalization and then use the subsequent rates of hospitalization, ICU admission, mortality as endpoints. That’s a more realistic use. We still haven’t sent KN95 masks to everyone who wants one. Tough to imagine the massive logistical challenges for widespread outpatient use can be overcome.
Looks promising, these results, but agree with Philip Lederer that i.v. administration is not ideal. How would you rate the place of remdesivir vs. fluvoxamine in treatment of covid-19?
In India,during our second wave in April 2021,we have used Remdesivir early on in high risk non hospitalised patients with amazing results,Since the hospitals were full,remdesivir was given in drive through clinics in some hospitals and we had paramedical team which visited the patients home for injecting it while we the doctors monitored the treatment on video consulation.
Have used fluvoxamine in 63 adult patients thus far (12/23/21). All “high risk” – metabolic syndrome, obesity, advanced age (>65), neoplasia, COPD, taking immunosuppressives. NOT prospective, randomized or controlled but good post-treatment follow-up by telephone, telemedicine or in-person (59!63).
All “recovered” – ie., no mortality, hospitalization, or clinical deterioration. Several with persistent (low grade) cough, “long Covid” (without definition it’s like pornography – can’t define.but I know it when I see it), anosmia/dysgeusia.
I’m impressed (and more available than Paxlovid as Omicron passes thru).
Could you clarify what dosage you have been using, 50mg BID or 100mg BID? I have seen both used in studies but I am a bit worried about side effects in the elderly population with the high dosage.
Interesting article. I hope the single dose therapy trial is held soon.
However, there is better news. Omicron has neither led to hospitalisation, sever disease, nor deaths. It is effectively beginning of the end of the pandemic.
International pharma lobby of Pfizer and Moderna :::
“Standardized Mortality Rates after dose 1 were 0.42 & 0.37 for Pfizer & Moderna,
and were 0.35 & 0.34 after dose 2”
UK has 12 confirmed Omicron deaths out of 45k confirmed cases..
*Fatality rate is 0.027%..
Fatality rate of 0.027%
This would get Omicron FDA approval.
Omicron is safer than Pfizer and moderna vaccine …
Omicrom seems to have put spanner in the vaccine companies plans.
Omicrom has got all characteristics of a successful parasitism. It grows in host exponentially, spreads faster, infects more people with case doubling time of 2 -3 days. In no time it will infect most of the population who can get reinfected after few months as it does not lead to very strong sterilising immunity. Previous vaccines donot prevent infection as evident from all initial cases were fully vaccinated air travellers.
But it also does not make hosts very sick nor kill (less than 1 percent mortality).
So no more fear of death from Covid or more suffering. Hence no more vaccine required as natural infections (asymptomatic or mild) will be rampant.
That effectively kills the golden egg laying goose for vaccine manufacturers.
No wonder they are bent upon selling as many boosters as possible while the sun shines (paranoia persists, fanned by Faucci and company)
Poetic justice, Faucci proposes, virus disposes.
Should not the enthusiasm here be muted by the fact that the results were not obtained against the Omicron variant?
Thanks Dr. Sax. I mostly agree with your points, as usual. However, I believe you missed the major caveat to the Pinetree study: there were only 17 total events of the primary outcome (2 in the treatment group and 15 in the placebo group). The results are extremely impressive, but there is a high likelihood that this trial overestimated the effect size of benefit due to random chance. This is a very common occurrence in trials stopped early, particularly in trials stopped early with few total end points. The perils of overinterpretation of effect size in trials stopped early were well described in BMJ 2012;344:e3863. The authors site three examples of the whole of medical practice being persuaded to adopt a practice based on an RCT stopped early with few outcomes, all of three of which with disastrous consequences.
Again, I mostly agree with you. I just think that given the mixed results you allude to in your essay in previous trials combined with the high risk of overestimation of benefit due to random chance, one must take the Pinetree study with a large pinch of salt.