An ongoing dialogue on HIV/AIDS, infectious diseases,
July 3rd, 2016
Velpatasvir/Sofosbuvir Makes HCV Treatment Simpler, Especially For Genotypes 2 and 3
One of the ways ID and hepatology hepatitis C experts like to show off is by discoursing on the nuances of cleverly named clinical trials, and how these impact treatment guidelines.
It usually goes something like this:
“In the EP-CILEON [I made that up] study of [insert HCV regimen here], treatment-experienced patients with genotype [insert non-genotype 1 patients here, usually genotype 3], compensated cirrhosis, and baseline viral loads greater than [some large number], the SVR [why can’t they say “cure”?] to the 12-week regimen was only [insert some number here that we could have only dreamed about in the interferon era, but well shy of the 95% mark we expect today — let’s say “82%”]. That’s why these patients need to be treated for [a longer duration than 12 weeks, a number also divisible by 4] weeks, with the addition of weight-based ribavirin [as opposed to fixed-dose ribavirin? when would we do that?].”
Fortunately, these obscure study results are then quickly incorporated into the excellent HCV guidelines, so we mortals can just look them up.
With the FDA approval last week of velpatasvir/sofosbuvir (VEL/SOF, “Epclusa”), however, bragging rights to these arcane details might now be irrelevant, kind of like knowing how to text with a flip-phone.
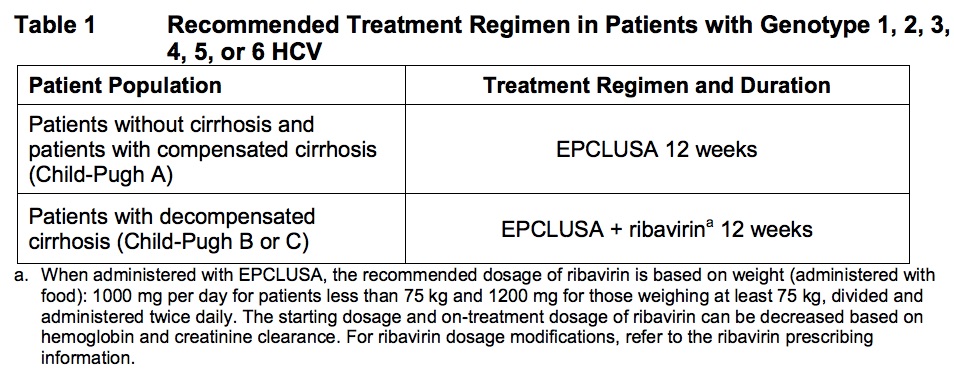
The new option makes things pretty simple, really. For patients with HCV genotypes 1-6 (that’s all of them, if you’re keeping track), here’s what to do:
- Almost everybody: One pill of VEL/SOF a day for 12 weeks.
- Those with decompensated cirrhosis (Child-Pugh B or C): Same thing, but add weight-based ribavirin.
Or if you prefer, from the package insert:
In several clinical trials (published right here in the hallowed pages of the NEJM), VEL/SOF cured 95-99% of those treated, including patients with tricky genotype 3. Side effects were infrequent, and, as we’ve come to expect with modern HCV therapies, discontinuations due to adverse events wonderfully rare (0.2%).
Of course you’re thinking — Sounds good, but what does this new treatment cost? Because that, after all, has been the major challenge in HCV therapy the past two years: Access.
The simple answer is that the price is around $75,000 for a 12 week course.
And that is potentially good news, as demonstrated by the following (approximate) list “prices” for various treatment courses, the most relevant comparisons for the VEL/SOF option bolded:
- Ledipasvir/sofosbuvir for 12 weeks, genotype 1: $94,000 (8 weeks for HCV RNA < 6 million, no cirrhosis, treatment naive, is 2/3 that cost)
- Paritaprevir/ritonavir/ombitasvir plus dasabuvir, genotype 1: $83,000
- Grazoprevir/elbasvir for 12 weeks, genotype 1: $55,000
- Sofosbuvir plus ribavirin for 12 weeks, genotype 2: $88,000
- Sofosbuvir plus daclatasvir for 12 weeks, genotype 3: $147,000
I wrote “potentially” good news, since these numbers are before negotiated prices. The negotiated price is the deal made between the pharmaceutical companies and the payers, behind closed doors and opaque to patients and providers, but quite relevant when we want to prescribe something.
Hardly any insurance plan or pharmacy benefit manager pays these full prices — it’s sort of like the sticker price on the car in the showroom, a starting point for discussion. However, it differs from buying a car in that there’s no easy internet research to find out what the plans actually pay. We prescribers and patients get a relative idea when the prescription either sails through or gets blocked in favor of something else.
But a quick glance at the clinical trials data and the sticker prices shows that VEL/SOF could easily become the recommended treatment for genotype 2 (good riddance, ribavirin!) and genotype 3. For the latter, this is not only based on price; sofosbuvir plus daclatasvir needs to be given for 24 weeks, with ribavirin, in all genotype 3 patients with cirrhosis. So VEL/SOF really is an advance — this is a simple, safe, and effective 12-week treatment for all genotypes that will rarely need ribavirin, except when there is decompensated cirrhosis (Child-Pugh 2 or 3).
And for those who are not very hepatically inclined, and wondering what the heck Child-Pugh is, it is a commonly used scoring system to assess the prognosis of patients with cirrhosis.
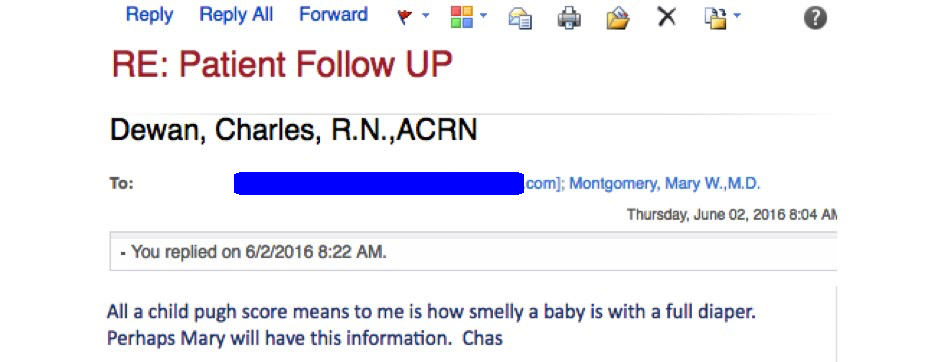
Take it from Charlie, our beloved clinic nurse, who had this email exchange recently with a pharmacy over getting HCV treatment approved for one of our patients:
Such a great line, had to share.
Hey, since I started this post with a parody of sorts, here’s one on TED talks making the rounds that could not be more spot-on.
[youtube https://www.youtube.com/watch?v=_ZBKX-6Gz6A&w=560&h=315]


Thanks for this, Paul. As soon as I saw the news about Epclusa’s approval, I thought, “Well, here’s a game-changer for Hep C treatment. But didn’t we just say that a few years ago when Harvoni was approved??”. (Who is in charge of thinking up those brand names, anyway?)
What is the rationale for adding ribavirin to the regimen in decompensated cirrhosis? Is it just considered a precautionary add-on due to the risk of treatment failure in these patients, or is there a reason I am missing? The package insert did not indicate why added ribavirin is added in these patients.
Loved the parody of the TED talks, some of which are quite insufferable.
Hi Loretta,
Not clear exactly what ribavirin does to increase cure rates for patients with cirrhosis, but it seems to be a consistent finding in clinical trials. One person explained it to me by saying that it “covers” the problem of pre-existing resistance variants to the NS5A inhibitors, but not sure mechanistically why that would be.
Such a mystery drug!
Paul
p.s. yes the parody is pretty brilliant, I agree!
Thanks! I am sure one of my brilliant (seriously) students will ask me this question when I teach this drug later in the summer. 🙂
Chas never disappoints.
I want brilliant funny blogs in rheumatology, cardiology, and endocrinology too~
Jeff, thank you for this nice comment!
-Paul
I second that emotion, and add neurology. And maybe, just maybe, psychiatry.
This made me laugh LOUDLY during my BIMA clinic. Thanks, Dr Sax!
Thanks for the article, Paul!
I am wondering, with the recent hoopla about pricing, we all know that despite this splash with a “low” price, as you mentioned, formularies rarely actually pay those list prices.
With that being said, at launch are net prices very similar to list prices? Presumably, this would make Epclusa’s actual price higher than Harvoni, which is quite heavily discounted now. Or do you think right out of the gate, Gilead has negotiated an even lower price with some payers that it would be on par with Harvoni?
Thanks!
Best,
James
The quick answer is that we (doctors, patients) don’t know. Plus there are as many payers in the USA as their are stars in the sky! However, I suspect you are right that LDV/SOF is now much cheaper for most patients than VEL/SOF, even more so if the 8 week option can be used (HCV RNA < 6 million, no cirrhosis, treatment naive). The real big difference will be between SOF plus RBV (geno 2) and, especially, SOF plus daclatasvir (geno 3).
Paul