An ongoing dialogue on HIV/AIDS, infectious diseases,
April 25th, 2021
The Decision on the Johnson & Johnson COVID-19 Vaccine Surprised Me — Here’s Why
The “pause” on the one-shot Johnson & Johnson (J&J) COVID-19 vaccine is over. Based on a further review of safety data that occurred on April 23, both the CDC and the FDA said the vaccine may resume here in the U.S., provided the label includes a warning about a serious, but rare, side effect — thrombosis with thrombocytopenia syndrome (TTS).
I confess this decision surprised me. My hunch was that they would advise limiting the vaccine in the U.S. to women older than 50, with no age criterion for men. Instead, it’s now available for all.
This was no doubt a tricky decision, one reflected in the 10-4 vote of the Advisory Committee on Immunization Practices (ACIP). When experts disagree, I find it useful to list those things we all can agree on:
- These are not your typical blood clots. TTS bears a strong resemblance to heparin-induced thrombocytopenia (HIT), with low platelets and development of antibodies to platelet factor 4. This “consumptive coagulopathy” has distinctive clinical features, is challenging to manage, and should not be treated with heparin — which can worsen the disease.
- The cases are very serious. Venous thrombotic events vary widely in severity; the clots in these TTS cases occurred in particularly bad anatomic locations, most commonly in the cerebral venous sinuses. Cerebral venous sinus thrombosis (CVST) can lead to permanent neurologic disability, require intensive care, or even be fatal. In the TTS cases, clots also occurred in other sites, and in the arterial system. From Friday’s ACIP meeting:
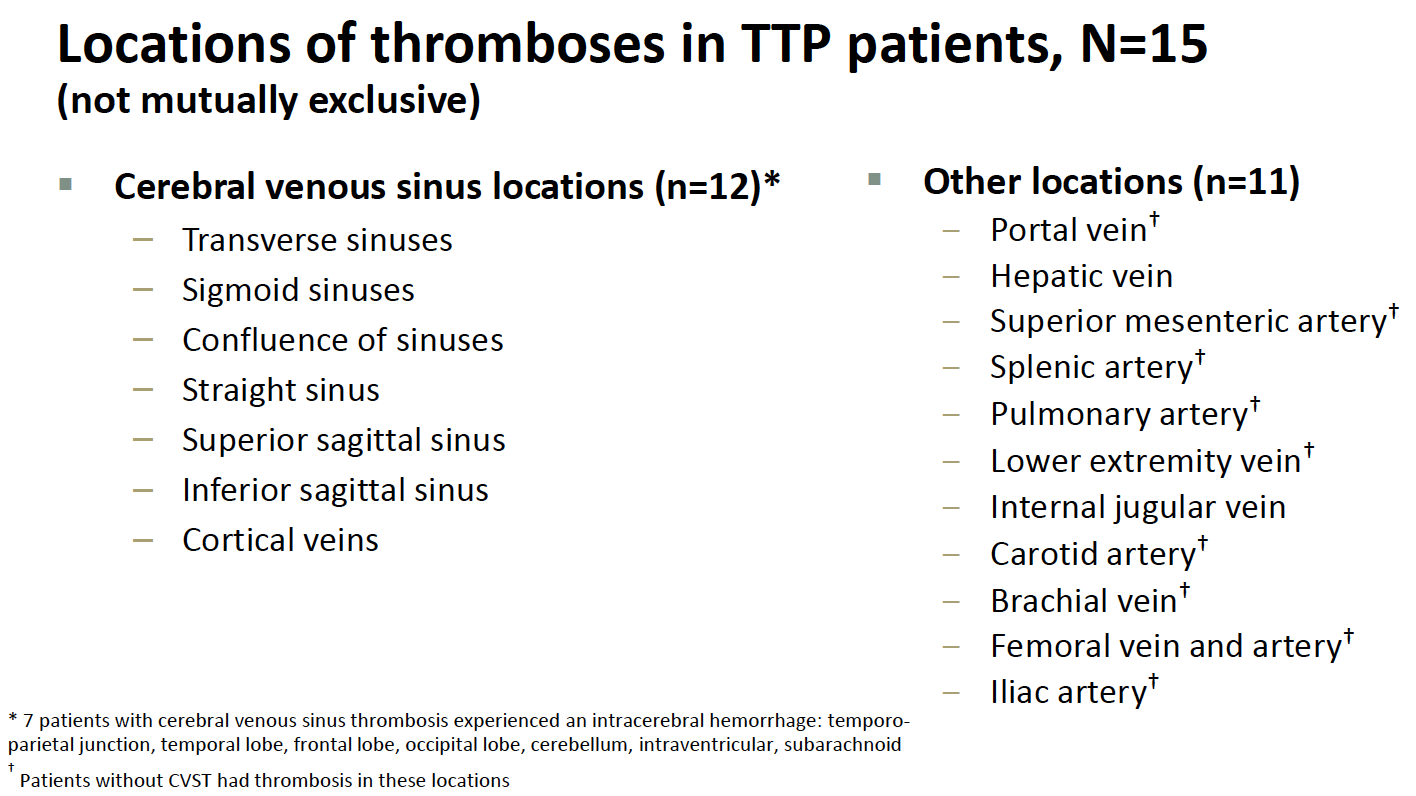
- They are rare. Nearly 8 million people have received the vaccine, and 15 of these distinctive clotting events occurred, for a rate of around 1 case per 500,000 people vaccinated. Additional cases may come to light, and apparently, around 10 are under investigation.
- The risk is higher in younger women. Thus far, all the cases occurring since the emergency use authorization (EUA) have been in women. The median age was 37 years (range 18–59). For women aged 18 to 49 years, the estimated TTS rate is approximately 1 per 140,000 doses — and potentially higher if more cases occur in this age group among the 10 or so being investigated. Plus, in hindsight, a 25-year-old man likely had a similar syndrome during the clinical trial.
- They occurred shortly after the vaccine. Median time to symptom onset was 8 days (range 6–15 days).
- Similar thrombotic events occurred with the AstraZeneca COVID-19 vaccine. This adverse effect is the primary reason some countries slowed the rollout of this vaccine globally or limited who should receive it. (The vaccine is not used in the U.S.) With the AstraZeneca vaccine, a broader demographic appears to be at risk. Both vaccines use an adenovirus vector strategy to deliver the SARS-CoV-2 spike antigen.
- No cases of TTS have yet occurred with the mRNA vaccines. While thrombotic events have been reported after the Pfizer or Moderna vaccines, none of the cases had low platelets or the other distinctive characteristics of TTS. Given the nearly 200 million doses of these vaccines already administered in the U.S. — with millions more globally — these are highly reassuring safety data.
- The J&J vaccine has several favorable characteristics. As a one-shot vaccine with less stringent storage requirements than the mRNA vaccines, it theoretically would be easier to give to a broader — and sometimes disproportionately at-risk — population. Specifically cited in the ACIP meeting included the homeless, rural residents, people in prison, disabled, homebound, or those with limited access to healthcare.
- We have a sufficient supply of mRNA vaccines to vaccinate all eligible adults in the United States. Given what India is going through right now, just writing this breaks my heart — but it’s true and must be considered in the risk calculation of using the J&J vaccine. Hence while the favorable characteristics of the J&J vaccine would make vaccinating the U.S. population easier, it may not be required.
- COVID-19 case numbers are falling in most of the United States. Even Michigan, the state with the recent marked surge, is fortunately now showing a sharp decline in case numbers. Again, this is critical when thinking about the risk calculation for someone choosing whether to be vaccinated and when.
The process by which safety issues come to light with these vaccines is truly impressive. These rare events triggered a thorough investigation, one in full public view with all the data shared. That’s a real win.
But let’s now consider an otherwise healthy young woman who wants a COVID-19 vaccine.
Give the availability of the mRNA vaccines, the falling case numbers nationally (and hence her reduced risk of disease), and the rare — but extremely serious — side effect of TTS that might occur with the J&J vaccine, under what possible circumstances should the J&J vaccine be the recommended approach?
If it’s a matter of convenience, I’d say it’s worth spending the time educating about how to get the two shots and avoiding the small risk.
If it’s a matter of lack of mRNA vaccine availability, I’d say fix the supply issue or outline where an mRNA vaccine is available.
But now? It’s possible that this young healthy woman might end up getting the J&J vaccine. And while the odds are overwhelmingly in her favor that everything will be fine, in practice, this would be giving a vaccine with a recognized safety issue when two highly effective and safe alternatives exists.
And the warning? I worry about women who lack the medical literacy to fully understand it, or the cultural authority to question what is being offered to them, or the forthrightness to request alternatives — or all of the above.
And that can’t be right.


You are my hero, Dr Sax! Thank you for your honest and conscientious comments. I agree that there should be age-criteria for the use of the J and J vaccine
Thank you for this enlightening and very articulate commentary, which reflects my own thoughts and concerns.
I think an important aspect of the debate is the reduced efficacy of the Johnson and Johnson vaccine, when compared to the mRNA ones.With an efficacy of around 66%, is it really worth using it instead of vaccines with an efficacy of 95%?
Hi Dr. Sax I agree with you 100% as always.
I think there is a typo above you have from one of the slides fromTom Shimabukuro, MD, MPH, MBA.
“Locations of thromboses in TTP patients, N=15”
TTP should read TTS.
That may cause a bit of confusion.
Thanks
Dave
Agree with Dr. Sax assessment. My reason. Rare event of thrombosis is in numbers only. But becomes reality when ONE person is affected by this rare event. Statisctics must fit with what happens in the real world. We don’t have that correlation here. Younger women should avoit the risk of thrombosis even if it is rare.
Thanks for pointing that out — the typo is on the slide as presented, hence I could not change it.
– Paul
Thank you, Dr Sax, for your frank and forthright critique of the current Covid vaccines.
I believe that the first rule in health care should always be “to do no harm”. However, it is my opinion that vested interests come into play regarding what type of vaccine has already been ordered by Governments. When there are two mRNA vaccines (Pfizer and Moderna mNRA) without the significant complication of thrombosis with thrombocytopenia syndrome (TTS), I believe it makes sense that they should be used instead of what appears to be riskier interventions for the public.
I agree with you Dr. Sax for the thoughtful comments. I think there may still be a role of the J&J vaccine, such as in situations where it is very difficult to give the patient a second dose etc.
A fantastic summary, as always. I agree entirely. I do not think the Janssen vaccine should be given to women age <50 unless receipt of one of the mRNA vaccines is not feasible (and as Dr. Sax points out, the reason for said infeasibility must be scrutinized, and efforts made to overcome it) and the potential recipient understands the risk. The likely lower efficacy of the single dose Janssen, compared to that of either two-dose mRNA vaccine regimen, at least vs B.1.1.7 and the dominant strains circulating in the US prior to the advent of B.1.1.7, also should be considered as we decide how to deploy this vaccine going forward.
I think the vaccine should go back to investigational status and check each recipient for platelet count, platelet antibodies, etc to see if there are subclinical cases.
I agree with Dr. Sax 100%! My 30-something year-old daughter was fortunate enough to get the Moderna vaccine. Given the efficacy, safety, & now availability of the Moderna and Pfizer vaccines, I do not see the justification of giving the J & J vaccine To reproductive-age women except in unusual circumstances such as hard-to-reach populations.
A very thoughtful response to the CDC and FDA decisions.I think many of us feel your discomfort, but few could express that discomfort so coherently.
I appreciate Dr. Sax’s common sense data-driven analyses. This & other articles he’s written. Thank you.
Thank you for your thoughts. I wholeheartedly agree!. Your comments are right on point and greatly appreciated.
Dr. Sax,
Thank you for writing this! As the Chief Medical Officer for my health system and engaging in a discussion with our system leadership regarding what to do with the J&J vaccine we currently have in our possession, I sent an email this morning (before reading your piece this evening – honest!) reaching exactly the same conclusions you did, and for precisely the same reasons. I am a bit dumbfounded by the decision to resume J&J vaccination without the limitations you suggest, and am grateful that you have publicly raised the ethical issues surrounding the efficacy of the warning.
Thank you for your continued thought leadership throughout the pandemic.
A thoughtful analysis and a convincing conclusion, articulated without ambiguity.
Completamente válidos todos sus argumentos Dr. Sax.
Estoy absolutamente de acuerdo con usted.
Es increíble que los CDC y la FDA hayan autorizado de nuevo el uso de esta vacuna teniendo ya tanta información sobre los efectos tan graves que puede ocasionar y sobre todo, como usted muy bien lo anota, existiendo alternativas para utilizar cómo son las vacunas de ARN mensajero.
Esperemos que las personas que van a vacunarse tengan a tiempo la suficiente información y se nieguen a aplicársela.
Lo felicito
I applauded Dr. Sax for pointing out that the risk of TTS/TTP is less rare in certain populations when we stratify the patient into subgroups. Instead of an overall incidence of 1 in 500,000 it became 1 in 140,000 among women aged 18 to 49. The real-life figure could be much higher if we were able to count the under-reported cases as well.
Now 18-49 is the child-bearing age and it is common for these women to take contraceptives. The association of contraceptives with thrombosis is well-known. When I worked in a vascular clinic in New Zealand in 1968-69 I was appalled by the number of cases with venous and arterial thrombosis among young women taking contraceptives. Since then they claimed to have improved on the dosage and formulation but the risk remained. A few years ago I had a 46 year old patient who, against my advice, had an intrauterine device eluting a contraceptive hormone (levonorgestrel) and she developed thrombosis of her left transverse sinus and left cerebral hemorrhage causing right hemiparesis. I wonder if the post vaccination subgroup at risk of TTS/TTP could be further narrowed down to those women on contraceptives?
I declare no conflict of interest.
And something more…
If we vaccinate the entire population with the Johnson and Johnson or Astra Zeneca vaccine, with a roughly 70% efficacy, that means that we expose the 30% of the vaccinated people to this serious side effect only, without any benefit
There is a common misunderstanding of the meaning of efficacy of the vaccine.
I hope the following explanation will help to clarify the situation.
The numbers J&J presented to the FDA on Feb 26th had randomized groups of over 19,500 vaccinated and an almost identical group given the placebo. The vaccinated group had 14 cases of Covid-19 that met the criterion of “severe/critical,” of whom only 2 required hospitalization, and none died. (All more than14 days after inoculation.)
For shorthand say:
J&J { 14 Cov / 2 Hosp / 0 Dead }. and for the placebo group the numbers were
Placebo { 60 Cov / 29 Hosp / 5 Dead } (All deaths occurred in South Africa)
The 14 versus 60 “severe” Covid cases was a 77% reduction in risk – ie 77% efficacy
(For cases occurring more than 28 days after inoculation, it was 85% efficacy. Only 2 months of follow-up were required – placebo people could then be offered the “real” thing, except that continuing surveillance is very desirable.)
The numbers of hospitalizations and deaths were too small to allow statistically meaningful comparisons. The confidence limits are too wide.
IF the group sizes had been 1,000,000 (not 19,500), AND the same ratios held, the numbers would have been:
J&J { 717 Cov / 102 Hosp / 0 Dead }
Placebo { 3,070 Cov / 1,484 Hosp / 228 Dead }
The misunderstanding I started with is that “30% of the vaccinated people ” are left exposed [while only 70% are protected].
(J&J 717) is 77% less than (Placebo 3,070).
30% of 1,000,000 would have been 300,000 — NOT 717
The other side of the coin would have been 700,000 — NOT 3,070
Brilliant! Excellent opinion piece. Share 100% all thoughts.
Thank you very much for your comment Dr. Sax. I completely agree with you, and at the same time I am surprised about the decision of the CDC and the FDA. I think the correct medical practice cannot be delegated to statististical criteria only.
Thank you again,
Vincenzo Marottoli
As I read the data on the J&J vaccine, I felt a tight knot in my stomach. Another reason for people to possibly avoid being vaccinated – damn.
Providing an intervention that could cause a serious or fatal complication is a very difficult decision. We make these decisions everyday in clinical medicine. Before deciding for or against this vaccine it would be fair and appropriate to understand the risk of TTS in the general population or with Covid-19. An “unpublished” retrospective cohort study from Oxford (yes I am aware of the perils) demonstrated a much higher incidence of cerebral venous thrombosis in Covid-19 than that of the vaccines (and both higher than the general public). Here again we are faced with a risk: benefit ratio to consider.
The world has and is facing a serious challenge.
I would favor a caution being placed on the middle aged female population for use of this vaccine
Al Taege.
Dr Sax,
Thank you for the brilliant explanation! It reflects my own thoughts! Your comments should be sent to all government officials in the world.
Your leadership and sound commentary during this pandemic is greatly appreciated. Greetings from Miami Beach!
Thank you for the consideration of opting to get Phizer or Moderna due to safety.A thought I had myself.
I disagree, strongly. The 1:500,000 risk you suggest is more than offset by the benefit of the vaccine. Every time any comment appears in the press another 10,000 or 100,000 people say “See, I told you these vaccines were risky”. Already we know that 20% do not return fore their second shot.
I volunteer at a vaccination center for the Forsyth Co (NC) Dept of Public Health. Three months ago, appointments were snapped up in minutes., Now we cannot fill even a small proportion of our capacity. Last Saturday, at the Fairgrounds we administered about 250 does. Our capacity is easily 2500. I have also participated on strike teams in church basements and in shelters. Getting people in for even one shot is a difficult task.
Because of vaccine hesitancy, we will not reach herd immunity except by infection, not vaccination.
There are other negatives as well. I suggest you check with radiologists and find out hoe many more (unnecessary) studies have been performed for headache following vaccination. The M and M of that greatly exceeds 1:500,000, not to mention use of medical resources.
These considerations need to be added to the decision making process. We are in an era of Public Health where broader thinking is required.
,.
I agree with Paul’s sentiments and conclusions.
Janssen/J & J vaccine is less immunogenic and less safe than Pfizer/BioNTech’s and Moderna’s vaccines. The serious safety events secondary to J & J vaccine are similar to those reported from AstraZeneca vaccine. Both are from the same adenovirus vector DNA platforms. So, there is some biologic plausibility. Proponents might have been able to argue for Janssen’s vaccine in February 2021. Now, vaccine supply does not appear to be as big an issue. So, supply/demand cannot be the reason for advocating Janssen’s vaccine in May 2021. I ask myself, if I have the choice of getting vaccinated between one of our current mRNA vaccines and J & J vaccine, without hesitation, the answer will be mRNA vaccine.
I will miss Paul E. Sax when NEJM ceases producing Physicians’ First Watch.
On point! I agree with your perspective and preceding comments. I was surprised the ACIP recommended a precaution over a contraindication. The severity of TTS as well as its presentation argue for a contraindication. Symptoms present not as a warning but rather as evidence of thrombosis. Then, the race is on for swift diagnosis and treatment. As I understand TTS, there is no other way to prevent this severe adverse event in a [rare] susceptible person other than to have the vaccine be contraindicated. At this point, the vaccine safety net seemingly needs to be cast population-wise.
One other brief thought is the pro-precaution argument is the J&J vaccine has utility (i.e., benefits outweigh risks) in rural areas and for at-risk populations, yet don’t those factors make diagnosing and treating TTS tougher?
Dr. Sax,
I agree with you completely 100% on this matter. An ounce of common sense is needed during these times. Common sense is on your side regardless of what researchers justify for the J&J vaccine.
I think when you normalize the risk to that particular age group and to the short amount of time that these events occurred and compare it with the risk of this demographic being hospitalized or dying from Covid, the risks may actually outweigh the benefits, especially considering we have a safe and effective alternative available. If it was the only vaccine available to me (a 40 year old woman), I would still get it, but would get an mRNA vaccine preferably if it was available. (For the record, I am already fully vaccinated and have been since January.)
Thank you for comprehensive simple language summary. Unfortunately, the understanding of the covid 19 crisis still seems to be significantly low among the general population. Indias devastating COVID 19 failed battle shows the risk of underestimating the virus. It has been painful to watch the suffering and loss that so many have endured. Why it is a cautionary tale to North America and the rest of the world. Despite investing millions of dollars to communicate the risk via public health messaging and are we communicating the real risk and danger of this virus to the general public the way they could potentially understand and in a realistic way? It is also very important that we understand how dangerous it is to disregard disrespect and undermine the power of our key tool against the covid 19. Rare blood clots related to vaccination should not be a deterrent to vaccination. According to the latest data 15 cases of 8 million vaccines and what is the real population risk? It is 0.0008% of all doses administered. We can understand that this rare clotting risk should not overshadow the benefits of COVID-19 protection. However, the key will be communicating to patients how to look out for a “one-in-a-million” side effect. American Heart Association cited the list of potential symptoms, which could occur up to two weeks after vaccination-blurry vision, fainting, sensory changes, seizures, leg pain or shortness of breath. Meanwhile, in a statement, the American Heart Association/American Stroke Association also asserted that serious complications from the COVID vaccine are very rare and urged people to get immunized as soon as possible. The European Medicines Agency, Public health Agency of Canada, US CDC and the FDA concluded there is a possible link between the vaccine and rare, unusual blood clots, and asserted to the general public that the benefits of the vaccine outweigh the very rare risks.
Our physicians and the health care community are well capable of early diagnosing and treating these conditions- rare blood clots effectively and efficiently. New clinical guidelines advise clinicians on what anticoagulation medications could use and what particular medications should not be used to treat this condition. More importantly, we have a better understanding of the clinical data, prevalence, specific symptoms and the population at risk. In public health, we always talk about the importance of prevention. Staving off the serious health issues related to this never-ending pandemic is utmost important.
Spot on, wise and sage advice. However the number of cases should be corrected for a denominator closer to 1.5-2 million for the women age 18-45 that got the vaccine. So 15 cases/1.5 million. Still exceedingly rare but a likely consequence of the vaccine for reasons stated above and similar experience with the AstraZeneca Adenovirus vaccine.
Also as Dr. Mike Osterholme has noted the incidence of CVTS in women with OCP or pregnancies and delivery complications is 10-30x higher than with J&J vaccination, and COVID-19 infections carry a risk that is 50x higher than with the vaccination. These are good references to quantify vaccination benefits and risks.
Thanks for the astute and balanced opinion.
I would also mention that studies need to be conducted to isolate factors predisposing to TTS amoung vaccine recipients, including OCPs usage; especially since most of the victims are females within reproductive age.
Thanks again.
10 people in 7 million with side effects is much less than the numbers of unvaccinated who would have died. More people probably got hit by a bus on their way to get the vaccine!
Thank you for the update.
I agree with Dr. Ross and Alan King’s second comment.
What is the risk-benefit ratio of a single-dose of an mRNA vaccine compared to a single dose of a vector vaccine? Because that is the real-world picture. Also, people–apparently including physicians–do not have a realistic sense of risk or understand the terms in a statistical sense. It is likely that the vector vaccines are a co-factor in a multifactorial scenario that leads to CVST. If one of the other co-factors were removed, would there be any elevated risk of CSVT with a vector vaccine?
A warning label is appropriate. There are absolute contraindications and relative contraindications. Potential vaccine recipients have varying reasons for choosing a particular product, and not all are based on lack of knowledge. Further, providers do not have to check their judgement at the door. You can make recommendations.
It seems counterintuitive that FDA/CDC allowed continuing use of the JJ vaccine. Evidently many experts have carefully assessed the risks and benefits and they did not just flipped a coin to arrive at this decision. Although I agree that when comparing JJ with the mRNA vaccines, it would be a no brainer to avoid JJ in the risk group for thrombosis, my hunch is that they took into account more than just the relative risk JJ/mRNA. If one places in the balance all the risks that a person will gladly take every day (driving, walking in the rain during storms, working, socializing with potentially infected persons, eating products with potential food-borne illnesses), I believe that the small risk of JJ vaccine would turn into a rounding error and irrelevant.
In Europe we have the same questions about the Astra Zeneca vaccine , although it has been indicated for people over 55 in many countries. But recent data show TTS is also present in people older than 55 ans and in men,just slighty less than in women. Another concern is the possibility of infraclinical cases, and the risk of more frequent and /or severe cases after the second injection .
Dr Sax words of caution with the Adenovirus vaccines are welcome ,I agree 100%
I thank you for your honest interpretation of the data. When I make similar comments, I receive angry if not threatening responses. A similar issue which deserves attention is the lack of agency recommendations on the risks or necessity of vaccines in people, especially women, with a documented history of previous COVID infection. Recent data demonstrates very little benefit with the second dose Also, the durability of antibody protection from infection is still unclear, and even the first dose may be able to be delayed for 6 months, a year, or longer. Many of these doses could be utilized in the third world where they are desperately needed. When the vaccine supply is abundant, we will have more data and see what the optimal timing is for vaccinating previously infected individuals.
My daughter in law came down with TTP and lost their 20 week fetus. We were afraid we would loose her too. Over 3 weeks at Swedish and some very expensive drugs she is doing well. It’s just been traumatic for the whole family.