An ongoing dialogue on HIV/AIDS, infectious diseases,
February 14th, 2023
Interferon Lambda for COVID-19 — Looking Good, but Still Not Available
Way back in the spring of 2022, I was asked to give an update on outpatient treatment of COVID to a group of general internists. The talk featured this slide on the TOGETHER trial of peginterferon lambda:
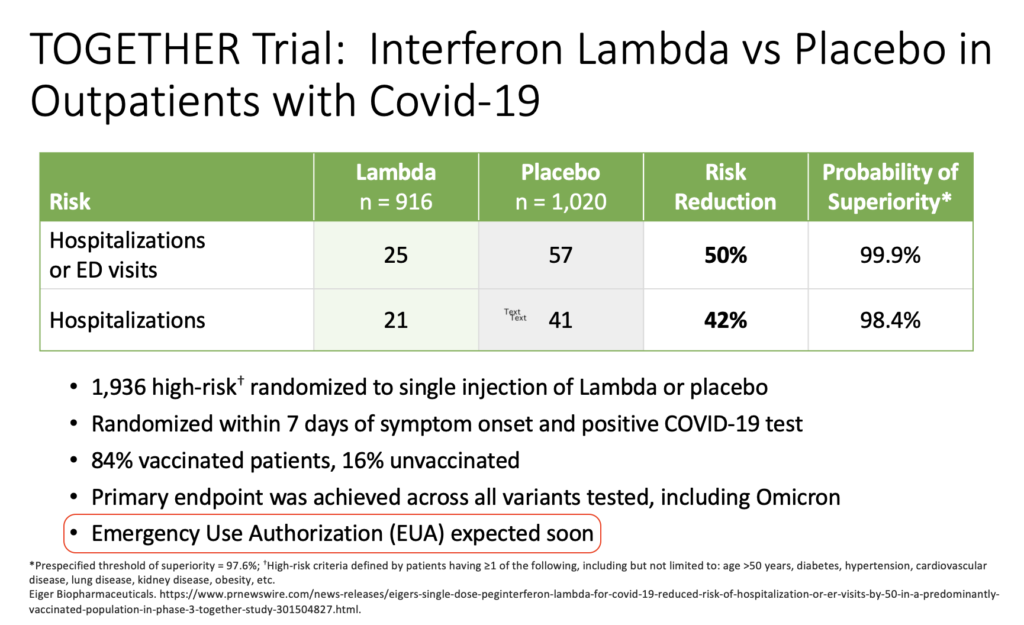
These data came from a press release from the company developing the drug. It’s dated March 17, 2022.
I added the highlight over the last bullet to make fun of my very bad prediction. Oops. Clinicians who treat COVID, or have known people treated for COVID (which covers essentially 100% of the U.S. adult population at this point), realize we still don’t have interferon lambda as a treatment option, now nearly a year later.
I summarized the reasons for my initial optimism in an opinion piece for the Boston Globe written with Dr. David Boulware (clinical trialist extraordinaire), namely:
- Efficacy shown even in vaccinated people
- Worked across all variants
- Dropped viral loads faster
- Side effects comparable to placebo
- “One and done” treatment
- No drug interactions
- Might work against other viral infections too!
Yep, the TOGETHER trial interferon results — published this past week in the New England Journal of Medicine — look really solid.
It’s not a perfect clinical trial. There were some issues with the drug supply during the study, and the blinding, and some have criticized the primary endpoint. There were apparently enough concerns that the FDA did not agree to meet with the company to discuss an Emergency Use Authorization. But all studies have weaknesses. I don’t think these are sufficient to invalidate the results.
Plus, it’s worth remembering that our current COVID treatments are hardly flawless. None of our treatments has documented efficacy in vaccinated high-risk outpatients. Other issues:
- Molnupiravir may not be effective at all — and has legitimate safety concerns.
- Paxlovid has a boatload of drug interactions and that annoying rebound syndrome that we still don’t know how best to predict or manage. Grrrr. (That’s annoyance.)
- Three days of intravenous remdesivir is cumbersome to set up, requiring either a visit to an infusion center or dedicated home care services, and hence is out of reach for many.
- Omicron and its subsequent mutations made all the previously available monoclonals inactive. So if you spent many hours learning how to spell (or pronounce) bamlanivimab, casirivimab, imdevimab, etesevimab, sotrovimab, tixagevimab, cilgavimab, or bebtelovimab, consider that a sunk cost.
I’ll acknowledge that reduced disease severity lowers the urgency of introducing a new therapy for COVID. Nevertheless, hundreds of people a day are still dying, and this virus isn’t going away anytime soon. Here’s a not-so-bold prediction — we’ll see a surge of cases pretty much every late fall and winter for the foreseeable future.
And having interferon available for COVID would greatly facilitate studying its use in other viral respiratory tract infections. Its mechanism of action, augmenting the host immune response to viral infections, could show activity across a broad spectrum of such pathogens — influenza (including H5N1) and RSV most notably, but even other common viral pests (metapneumovirus, rhinovirus, the pre-SARS-CoV-2 coronaviruses). I contacted the TOGETHER study’s senior author, Dr. Jeffrey Glenn, who wrote:
I have been advocating for a trial I call the RELIEF (REspiratory viruses treated with Lambda IntErFeron) study, where patients who present with acute respiratory symptoms are immediately randomized to lambda or placebo, and we sort out later what virus they have. This could generate more data in COVID, but importantly advance the ball further by generating new data for other viruses of pandemic potential. We could leverage the same great infrastructure of the TOGETHER trial and hopefully generate game-changing data.
So, here’s hoping this promising and novel therapy gets another chance, perhaps in this confirmatory study.
Oh, and by the way, my Globe piece prompted this email from my daughter Mimi:
Go dad!! Great title.
Yep, it’s one of my better ones.


Are the FDA planning to review these data now that the paper has been published?
Very interesting.
The interferon looks too good to be true specially as a panacea for variety of viral infections.
I am afraid:
1. Are we missing something, that is what FDA perhaps thinks and is cautious (a la Thalidomide in pregnancy). The watchfulness could avoid possible but unexpected harm.
2. Better planned and conducted study is needed.
3. If it is so useful and effective, will the Pharma companies allow it in market as it could effectively wipe out their profit margins.