An ongoing dialogue on HIV/AIDS, infectious diseases,
October 18th, 2020
Does Remdesivir Actually Work?
Quick answer — it’s complicated.
Let’s start with a clinical anecdote — rightfully considered the weakest form of evidence, yet paradoxically holding great power over us because we’re imperfect humans. It’s the way we’re wired.
In April, a patient of mine with stable HIV came into the hospital with COVID-19 pneumonia. (Certain details changed for privacy.)
She works cutting hair, in a community hard-hit from the pandemic and where mask-wearing was inconsistent. She knew as soon as she developed fever, chills, and back pain that this is what she had.
She must have had symptoms for no more than 36 hours when she arrived at the hospital, and because she has HIV (who knew whether this worsened outcomes?) and is quite overweight, she was admitted.
She enrolled in the SIMPLE study — which compared remdesivir for 5 or 10 days to standard of care, all open-label — and was randomized to the 5-day course. She received her first dose of the drug the night of admission.
Again, she had been symptomatic for no more than 2 days.
The next day, she looked like a new person. Her fever was down, she was breathing more easily, and she told me her back pain went away as soon as the first dose had completed its infusion. She left the hospital on day 3, and made a complete recovery.
Of course, she could have recovered just as quickly without remdesivir — that’s the problem with an anecdote.
But based on this and other cases I saw — and the extensive experience of my indefatigable colleagues Dr. Francisco Marty and his team, who enrolled dozens of patients into this study — I was not surprised when in May, a different and more rigorous remdesivir clinical trial reported significantly faster recovery in the treatment arm than in the controls.
And because this study — called ACTT-1, now with final results — included a placebo arm and was blinded, this provided much stronger evidence that remdesivir actually works. (That’s in the title of this post.) It worked particularly well in people with shorter duration of symptoms and in those requiring oxygen. It didn’t help people so sick that they needed mechanical ventilation or ECMO.
When the data from ACTT-1 became available, we created a construct about these critically ill patients who didn’t benefit from remdesivir. In this view, they were in the immune phase of the illness, where the body’s immunologic response to the infection drove more disease than viral replication.
We can’t expect an antiviral to control these processes. Just like oseltamivir or baloxavir for influenza, you have to act early with remdesivir when treating SARS-CoV-2. Let dexamethasone or some other immunomodulator do the late work.
See, it all fits together perfectly.
But there were always holes in this neat little package.
First, the original remdesivir study from China showed no benefit of treatment. Yes, it was underpowered due to dropping case numbers, but the drug didn’t lower viral loads in recipients either. Concerning.
Second, the open-label study my patient enrolled in had a funny result in the 10-day arm — no apparent benefit compared with standard of care. (The 5-day arm did show benefit.) How do we explain that?
Now we have the interim results of the SOLIDARITY study, at least in preprint form, and that neat little package has even more holes.
In SOLIDARITY, over 11,000 hospitalized patients with COVID-19 (from 405 hospitals and 30 countries) were randomized between whichever study drugs were locally available and open control. This included up to five options — four active treatments versus local standard-of-care. The drugs were lopinavir/ritonavir, hydroxychloroquine (remember those?), interferon beta-1a, or remdesivir.
(Based on the limited availability of some of the drugs across countries, the study arms differed in size.)
The results for all the interventions failed to show a survival benefit. And the survival curves for the remdesivir arm versus standard of care look depressingly the same:
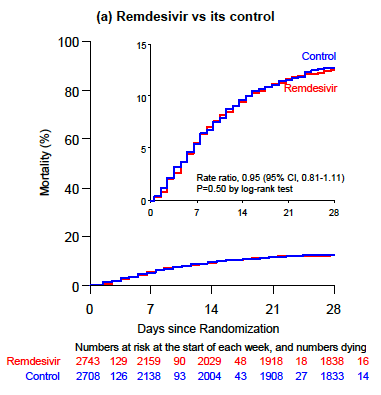 You could barely draw curves that overlap so precisely. This figure has been emblazoned on the retinas of ID clinicians since the preprint was released last week.
You could barely draw curves that overlap so precisely. This figure has been emblazoned on the retinas of ID clinicians since the preprint was released last week.
So how do we explain these discordant results? We can’t do so completely given the different study designs and populations. But just as how ACTT-1’s benefits can’t overrule the SOLIDARITY results, nor can SOLIDARITY negate ACTT-1 or the 5-day results from SIMPLE.
So let’s put all the studies together, as shown here in this colorful meta-analysis, and exclude patients who are on mechanical ventilation since no study demonstrated benefit in this population:
https://twitter.com/mikejohansenmd/status/1317056317522169856?s=20
First, we’ll note that SOLIDARITY’s much larger sample size trumps (ouch) the other studies. Second, the point estimate just crosses 1 (no benefit), but falls to the left of the line, suggesting (if you squint) some benefit.
If I had to postulate where we’d see the greatest benefit for remdesivir, it would be in patients with shorter duration of symptoms. Even in the negative underpowered study from China, those with fewer days of illness did better than controls. Could it be that the favorable results in ACTT-1 were from the fact that 25% of patients were enrolled with symptoms for 6 days or fewer?
Related, SOLIDARITY began enrollment in March, and for much of the enrollment period, patients with COVID-19 did everything they could to avoid hospitalization. For many, I suspect the short window of time for this antiviral to benefit had closed by the time they were admitted. Duration of symptoms is not reported in the preprint, a critical piece of information.
So for now, the answer to the question, “Does remdesivir actually work?” is a cautious maybe. Sometimes. For some people.
Which, given the absence of anything else right now and its low toxicity, means I’d still recommend it for most hospitalized people with COVID-19 — with the hope of giving it sooner rather than later, especially for those on oxygen at high risk for disease progression.
But if we can learn anything from the mental gyrations required to square these conflicting study results, it’s that we definitely need more effective options.



A stich in time saves nine. As with many other anti microbial drugs, Remdesivir too can act on its target at appropriate time, and that is in the early phase of disease/infection. The second half of Covid (beyond 7 days) is a different story all together, scripted with inflammatory molecules and collateral damage to the tissue. Having crossed the critical point of no return, the antivirals will not play any role in recovery i this phase, the immunomodulators perhaps can.
Having an arsenal is important but critical is the knowledge when to use which one. Remdesivir upto 5-6 days of symptomatic phase, Dexamethasone and other immunomodulators from 7th day onwards.
In each drama, the actors have specific role to play at specific time.
Given the pharmacology of remdesivir and the nature of COVID-19 disease progression, it “makes sense” that it would have its greatest affect early in the disease process. Given the immunologic up-regulation in the mid-to-late stages of the disease, where steroids are most effective, early diagnosis and treatment with remdesivir may prevent or attenuate disease progression.
It’s an antiviral acting on a short-lived viral disease; of course it’s the days post symptom onset that is driving discrepancies between the studies. SOLIDARITY, despite being an antiviral trial, failed to record data on days post symptom onset. For ACTT-1, it was a median of 9 days, range 6-12 days, which is still a shockingly long amount of time to being antiviral administration. The open-label mild/moderate trial was median 8 days, range 5-11 days.
The most promising trial of remdesivir is a double-blind placebo trial of outpatient remdesivir currently underway, which starts administration < 7 days post symptom onset and < 4 days PCR positive, and has a reduction in hospitalization as the primary endpoint and viral loads as a secondary endpoint. We will likely soon know empirically, not just theoretically, that remdesivir works earlier in the course of infection.
Dear Dr Sax My experience with COVID 19 diseased patients has been similar .We give Ramdesvir to all patients other those that are mildly symptomatic as part of a protocol recommended by WHO. The results are gratifying .I must concede that Dexamethasone given concurrently could have an additive effect The WHO has since revised its recommendations but wee prefer to continue Ramdesvir.
I think an antiviral works best at the beginning of the illness. After a few days of COVID doing damage to the lungs, the drugs don’t help as much.
The analogy is a flood in your basement. If you actively pump out the water before it gets into the bsorbent materials of the house, you can benefit from pumping and save the house. On the other hand, if you start pumping 5 days after the flood, your house has already sat in water and wood swelled and got moldy. Pumping so late has not saved the house.
WHO is too hurry to denounce an important therapy without carefully analyze its data with common sense. Just as it did many missteps in this pandemic.
Am an internal medicine resident in one of Venezuela main teaching hospitals.
We have been using Remdesivir combined with LMWH and Steroids and we’ve been getting positive outcomes..
Nonetheless it will be quite necessary to make a follow up on this study in order to determine which of those medications actually yielded to a positive outcome of critically ill covid patients.
Would one ask Sachin Tendulkar to open the innings or come to bat at number 4 in a T20 or one day match as compared to a test match? When we know the virus starts replicating even when one is asymptomatic, why not give a direct acting anti-viral agent (DAAVA) right on day one of diagnosis and check contacts so that if thy are positive but asymptomatic or presymptomatic, they would also need to be treated with a DAAVA. Hit hard hit early. Don’t wait to initiate with a DAAVA till it is too late. This way you check the virus and give the immune system a chance to catch up. Else the immune system can become dysregulated leading to a cytokine storm. Stop spread and progression. Now remdesivir has been emergency use authorised (EUA) for patients hospitalised with moderate COVID-19 too. Later when an inhaled version comes, it may be EUA even for mild cases. The earlier the better. You don’t get a second chance to make a first impression.
What is role of Remdesivir in cancer patients?
Does coexistant Diabetes Mellitus predispose to TB?
The chance that Remdesivir works is indeed debatable. Thanks for your critical comments. A primary endpoint as has been chosen in the NEJM papers aiming at hospitalization and not at complete recovery is debatable too. Patients with Covid-19, who still required oxygen supply after discharge from the hospital were included into the group of patients with a benificial effect under Remdesivir treatment. Hospitalization time must not be confused with cure. In addition hospitalization time in an international study is not a strictly defined end-point and varies all over the world even in Corona times. We urgently need better drugs.
According to my limited experience, patients treated with Remdesivir had reduced oxygen requirement and clinical improvement was seen.
I do not recall the same willingness to explain discordant findings about hydroxychloroquine effectiveness, which(if any) is also supposed to be early in the disease.
In our experience it works. We base its use in moderate cases when there is increasing O2 demand and has increased inflammatory markers and High CT scores.
Mohanty (india)
On the anecdote: was the patient PCR-tested?
Dexamethasone is an immune suppressant and not an immune modulator.
Covid-19 affects those who are borderline Lupus, and don’t even know it.
I am a provider who contracted bilateral covid pneumonia post right TKA. Was admitted for acute respiratory distress, fevers of 104. Pulse ox initially 50’s then to 70’s and eighties with high flow oxygen. My medical team initiated remdesivir with steroids, iv antibiotics and IV IGG plasma. This treatment saved my life. We all must understand each patient and their conditions vary but in my case it was totally appropriate care.
Totally agree, Dr Sax. And thank you for the reflections
Dr. Sax. I agree with your conclusion. HOWEVER, there is a big assumption being made here. Neither ACCT or SIMPLE provide any virology results. It is all symptomatic. To reach some of the conclusions you make, you need to show viral levels. There is one study that shows that remdesivir does not reduce viremia compared to placebo. The virology data should eb available as it was captured as part of the protocol. Disclosing the results should be extremely valuable and urgent. Lastly, we don’t know if the SOLIDARITY trial is useful. I am not aware of other trials utilizing that design, with such limited data being gathered. Also, the mortality is highly uneven, Latin America’s death rate is above 20%!
“…sample size trumps(ouch)…”
Was that really necessary? Am so disgusted when these medical articles needlessly become a vehicle for venting ones political angst.
Dear Dr. Paul, Your blog remembers me the experiences from the early years when I started my HIV Medicine practice in South India.That time we had toxic drugs which were recommended in the late stages of the disease and we could see the outcomes later when we had safer drugs which could be used in early stages of the disease. You yourself had commented on the Namsal trial in your blog previously. I had similar experiences like you when I started treating Covid-19HIV infected and non- infected patients. I myself survived because of remdesivir when I contracted Covid-19 and developed both pneumonia and shock on the 4th day of illness
Very good article. Remdesivir is just like Tamiflu or many other antivirals earlier the treatment (within 72 hours) better the results.
After 72 hours, we are treating inflammation more than infection. Anti inflammatory, IV antibiotics, IV immunosuppressant and plasma IGG are better options (Heparin if labs suggest the use)
Pharmacist‘S point of view.
All of us who treated and still treat influenza know very well that its antivirals have to be started within 48 hours, and we all select those who are the sickest in those 2 days to give such treatment. I am still unsure why this principle has not been utilized in clinical trials as an entry criterion, i.e. those who present with the severest symptoms from the outset esp those above 40 years and/or have comorbidities. I expect that we will see benefit in 1) shortening the symptomatic phase, 2) decreasing the rate of complications seen in many untreated patients (so-called “long haulers), 3) decreasing rate of hospitalization, 4) decreasing need for ICU care, 5) decreasing mortality. If some or all of these end-points are proven, then we shall see results that match what we have seen with anti-influenza antivirals.
The benefit seen in the only fully powered randomized placebo controlled study showed benefit, albeit modest, but with a p value (<0.001) that would rule out chance as a the reason for the effect. ACTT-1 reflects the types of patients coming into hospitals in the US. Reduces time to recovery by 4 days. Modest but a clinically meaningful improvement.
Is Remdesivir indicated in a COVID 19 patient with ground glass opacities on CXR and normal O2 Sats? Should inflammatory markers influence decision to start RDZ?
If it’s an effective antiviral drug, why no affect on viral load in initial study from China, and why no viral load data from any other human study? Imagine approval for any other antiviral during current times without that data?
Remdesivir really works well in early phase of illness with lung involvement and desaturating patients.
Definitely with steroids.
I have already treated 2250 plus cases till now with my team.i definitely feel Remdesivir helps given first 2-3 days of onset of symptoms especially in those patients with comorbids like age more than 55 yrs, DM, HTN , PLHIV, pts on cancer chemotherapy
SOLIDARITY study was more of a pragmatic study , which reflects the real practice , Remdesivir use is now more debatable , we may need to consider if there is benefit of combination or newer treatments instead of looking for treating earlier which is not realistic considering the enormous number of outpatient COVID patients
There are confusions about the efficacy of REMDESIVIR in medical community and researches. Taking a drug as magic cure for something without considering its mechanism of action is not only simplistic view but also unprofessional attitude.
REMDESIVIR mode of action is as an adenosine nucleoside triphosphate analogue, similar to FAMCICLOVIR as an a guanosine analogue antiviral drug. The later drug is only given within 72hrs of the HSZ infection, to stop spread of the virus beyond its irreversible damages, hence REMDESIVIR also should be used in early stage of COVID-19 to be effective not in later or even mid stages of the disease in order to do its job. If you give it later stage it is like giving FAMCICLOVIR in HSZ patient with well developed rashes and expecting to be effective.
What I cannot understand is how, in light of the Solidarity study, the FDA has given full regulatory approval to Remdesevir. It seems the ACTT study was enough for the FDA without resolving the conflict between the trials. This is going on at the same time as the FDA is publicly saying it will not approve a SARS-CoV-2 vaccine (even though several other countries are already immunizing their populations),and not even consider an EUA, until they have the most robust of large scale studies clearly proving efficacy and safety. The only common theme among these is Gilead, the manufacturer of Remdesevir, with a $1.6 billion government contract before final approval was granted. The head of the DSMB on the SARS-2 vaccines is reported to have been paid almost a half million dollars by Gilead last year. Why was Remdesevir granted final approval while the desperately needed vaccines are being withheld?
Dr Sax,
thank you for your remarks and thanks to other clinicians who have commented, several supporting early use of remdesivir infusion in treating Covid-19, the earlier done the better being the outcome.
As a former lead virologist for the British Public Health Laboratory Service, I’d like to suggest that timely remdesivir may also diminish infectiousness with benefit towards health care workers and other contacts.
WHO seems to have drawn a premature conclusion about remdesivir which may have an important role in the immediate management of suspected SARS-2 infection, at least in the vulnerable..
.
The SOLIDARITY trial is more reflective of the reality faced by clinicians practicing in the third world where the drugs to be used are severely liimted by supply issues and the patients come in different stages of their illness. More often than not, patients come to the hospitals after so much delay because they do all they can to avoid doing so for various reasons. In contrast, the ACTT-1 trial may be more reflective of the practice in first world countries.
I was just wondering why the USFDA did not take the results of the SOLIDARITY Trial into consideration when remdesivir was approved for use in patients =/> 12 years old last Oct. 22, 2020.
yes, REMDESIVIR shortens the hospital stay but seems to have no mortality benefit.
Good post! We will be linking to this particularly great post on our website.
Keep up the good writing.