An ongoing dialogue on HIV/AIDS, infectious diseases,
February 12th, 2022
The Rise and Fall of Ivermectin — 1 Year Later

Cheese consumption and death from bedsheets are highly correlated.
Here’s a confession few board-certified ID doctors will make — there was a brief period when I thought ivermectin could very well be an effective treatment for COVID-19.
It wasn’t when the in vitro data first came out. Therapeutic concentrations were not achievable in humans.
Nor when the anecdotal reports started pouring in, and sometimes making news. A former colleague of mine, a smart and clinically active person practicing in the Midwest, contacted me in late 2020 telling me that they acted as the primary medical consultant for a nursing home. Since they started using ivermectin, no patient had died or even been hospitalized from the disease. OK, a hopeful observation, but not proof.
Not when the figures appeared correlating ivermectin use and lower death rates from COVID-19 in some countries, mostly from tropical regions in South America and Asia. These figures always reminded me of these spurious (and often hilarious) correlations. Did you know that per capita cheese production correlated strongly with the number of people who died by becoming tangled in their bedsheets? Who knew? And what’s the mechanism?
And certainly not when these folks started pushing ivermectin with an enthusiasm that is frankly religious in intensity. This group’s treatment “protocols“, with their hodgepodge of antimicrobials (including ivermectin), immunomodulators, and vitamins — the kitchen sink approach — strain credibility.
Nope — my moment of greatest hope for ivermectin came just over a year ago, when Dr. Andrew Hill presented results from a meta-analysis of several randomized controlled trials, a presentation he would later also give to the NIH Guidelines panel. Andrew is a well-respected clinical researcher, someone well-known in the HIV research world.
In his analysis as first presented, the risk-ratio for mortality with ivermectin was 0.17 (95% confidence interval 0.08, 0.35), an 83% reduction in risk for dying. Outcomes for other endpoints (time to viral clearance, time to clinical recovery, duration of hospitalization) also favored treatment over controls.
Hearing these data, I reached out to Andrew to discuss his findings, and he generously discussed them with me. He acknowledged that the data were incomplete, but remained strongly suggestive of clinical benefit. Furthermore, he’d been in direct contact with the researchers who conducted the largest of these studies. They repeatedly reassured him that the data were sound.
I subsequently summarized my thoughts here in a post entitled, “Ivermectin for COVID-19 — Breakthrough Treatment or Hydroxychloroquine Redux?”; writing:
My take-home view? The clinical trials data for ivermectin look stronger than they ever did for hydroxychloroquine, but we’re not quite yet at the “practice changing” level. Results from at least 5 randomized clinical trials are expected soon that might further inform the decision. NIH treatment guidelines still recommend against use of ivermectin for treatment of COVID-19, a recommendation I support pending further data — we shouldn’t have to wait long.
What happened next? I offered Andrew the chance to submit the meta-analysis to Open Forum Infectious Diseases, which after conducting some additional analyses, he kindly did. Remember, at this time in early 2021, we had no readily available effective outpatient treatments COVID-19. Something inexpensive, safe, and widely available certainly would be most welcome.
After peer review and some revisions, with Andrew and his team reducing the survival effect size for ivermectin to 56% (still highly significant) due to some additional studies, we accepted the paper. We simultaneously solicited a thoughtful editorial from my Boston ID colleague Dr. Mark Siedner, entitled “Ivermectin for the Treatment of COVID-19 Disease: Too Good to Pass Up or Too Good to Be True?”
Well, we now know that the second part of Mark’s brilliant title turned out to be the case — too good to be true. Many of the studies on which the meta-analysis was based were highly flawed, and one was outright fraudulent. The fake data problem came to light just shortly after the meta-analysis appeared in print.
To his credit, Andrew promptly contacted me when the news broke. He immediately offered to retract the original paper, and even better to submit a detailed analysis of what went wrong.
This revised paper has just been published, entitled “Ivermectin for COVID-19: Addressing Potential Bias and Medical Fraud.” It includes this extremely telling figure, which shows how the effect size of ivermectin on survival drops to meaningless with exclusion of the fraudulent and potentially flawed studies:
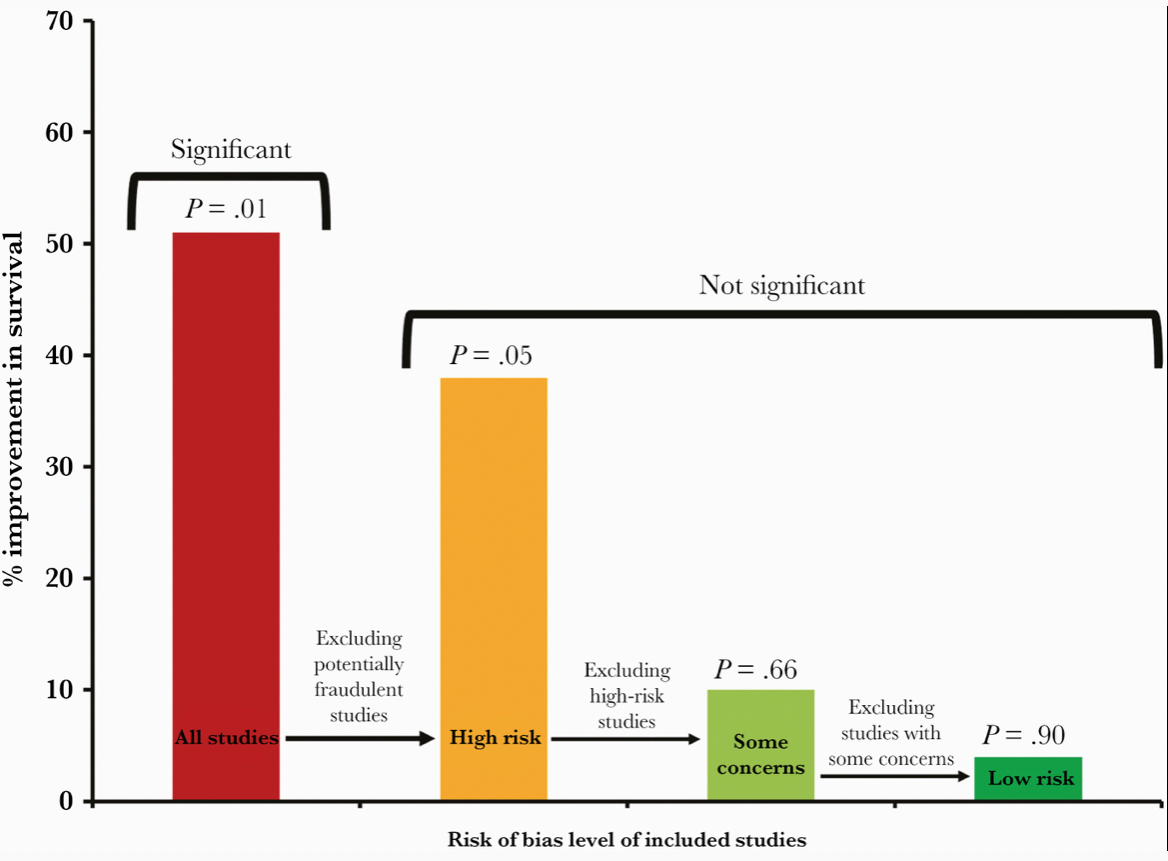
Read the full paper! But if you don’t have time, here’s the lesson:
These revised results highlight the need for rigorous quality assessments, for authors to share patient-level data, and for efforts to avoid publication bias for registered studies. These steps are vital to facilitate accurate conclusions on clinical treatments.
Indeed. And for whatever role I played as an editor in sustaining the confusion — and sometimes heated conflicts — over this potential treatment for COVID-19, I deeply regret it and apologize. I hope this post, and even more, Andrew’s revision, explain what happened, and hope we all can learn something from this mistake. Certainly I did.
Meanwhile, several large placebo-controlled trials continue to test ivermectin for COVID-19 treatment in outpatients. It still might do something (though at this point I’m not very hopeful). At least one of the studies — COVID-OUT — is fully enrolled, and ACTIV-6 is far along, so more data will be forthcoming soon.
Will these results be enough to quell the controversy about whether ivermectin has a role in treatment COVID-19, settling the issue once and for all? Let’s hope so.


Excellent summary and analysis of what happened. Thank you
I humbly agree with Dr. Adams. There is nothing to forgive. Only praise.
This is really weak. “low risk” Fonseca study; very sick patients, only 2 days of IVM at a dosage that is a fraction of the dose recommended. Lopez-Medina: mild symptoms for IVM and control. 37 median age. IVM symptom-free 10vs.12 day for placebo.
The two “low risk” studies bias the results. One provides low dose IVM to severely sick patients, the other treats a group of younger patients with mild illness that would likely all improve in a short time regardless and shows that IVM pats improved in 10 days and placebo in 12. Conclusion: IVM has limited to no effect. I think that’s lame.
There’s nothing I like better than watching scientists follow the data. Andrew’s data led him in one direction earlier, and a revelation about that data led him elsewhere later. This is science cranking its gears in good working order! Kudos to both Andrew and Paul.