An ongoing dialogue on HIV/AIDS, infectious diseases,
October 29th, 2015
The Most Important HIV Study at IDWeek 2015
After reporting my choice for the most important HIV study at ICAAC, I received this email from a colleague:
If that’s the most important study, I really didn’t miss much …
Now she has notoriously high standards — hard to impress her — but her opinion notwithstanding, I still think the STRIIVING study has some important messages we can apply to clinical practice today. So I stick by my choice.
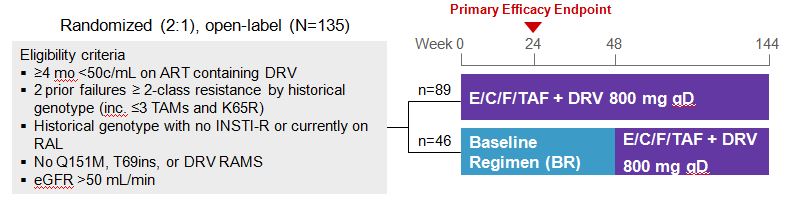
Now let me see if I can meet her approval with an IDWeek 2015 (which took place earlier this month) selection. It’s another HIV switch study, although this time with a twist. Instead of the usual switch-study population (first regimens, or only those with no history of virologic failure or resistance), it looked at a very different group — patients who were virologically suppressed but highly treatment-experienced, all with 2-class (or more) resistance, and taking multiple pills a day with a darunavir-based regimen.
Subjects were then randomized to continue their current therapy or switch to the two-pill treatment of elvitegravir-cobicistat-FTC-TAF (ECF-TAF) plus darunavir 800 mg, both once daily. (Thanks to Greg Huhn, the presenting author, for sharing the slides.)
At baseline, the regimens included a median of 5 pills, and around 60% were taking at least 6 or more pills a day. Most had a history of M184V, and a significant minority had K65R and/or PI resistance.
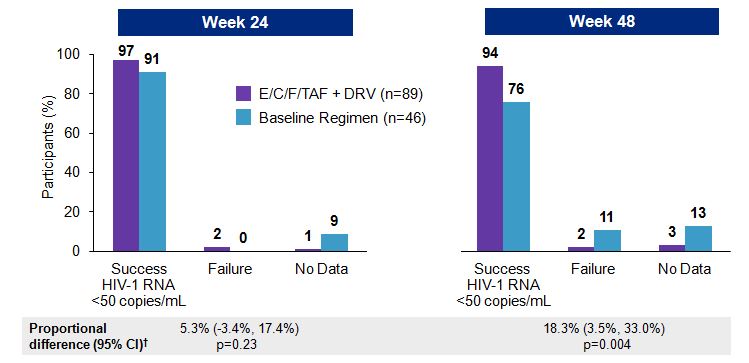
At week 24, 97% vs 91% of the ECF-TAF + DRV vs continued baseline regimen groups had HIV RNA < 50 cop/mL; at week 48, it was 94% and 76%, a significant advantage of ECF-TAF + DRV.
No new resistance was observed in those with virologic failure on the 2-pill arm; one subject developed resistance in the baseline regimen group, but not integrase resistance. A PK substudy demonstrated acceptable levels of both EVG and DRV, greatly exceeding IC95 and IC50 respectively.
Some comments on this study:
- Approval of ECF-TAF is expected soon, and I suspect this particular indication for switch will not be part of the product label. The data are very recent, and the study is relatively small.
- Even if it’s not in the label, however, these study results certainly suggest that some patients on complex regimens (many of whom are yearning to take something simpler) will be able to shift to just two pills a day — a welcome change.
- The PK substudy was extremely reassuring, greatly diminishing concerns about the head-spinning three-way interaction between elvitegravir, cobicistat, and darunavir. That interaction, by the way, is the reason why we shouldn’t use darunavir with the currently available ECF-TDF.
- Take a close look at those eligibility criteria — in particular the resistance ones — because they’re important. It’s why I italicized the words “some patients” in my comment above.
- Why is this so important? Patients with more than 3 TAMs, the multi-NRTI resistance mutations Q151M or T69S, or darunavir mutations were excluded. We don’t know if switching patients with any of these criteria to ECF-TAF and darunavir will maintain virologic suppression.
- Virologic rebound for patients with multi-class resistance who are stably suppressed could be disastrous — it would be terrible to take a patient with 3-class resistance and virologic suppression to 4-class resistance (including integrase) and rebound, especially since the HIV drug pipeline has relatively few investigational agents.
The take home message from this study is that when ECF-TAF is approved, a subset of our patients who are taking complex regimens could safely switch to two pills a day — ECF-TAF + darunavir.
Finding the right patients will take meticulous scrutiny of their historical genotypes. But of course that’s why they pay us the big bucks!
Next year’s meeting? October 26-30, New Orleans.
[youtube http://www.youtube.com/watch?v=pXHdqTVC3cA&w=420&h=315]