An ongoing dialogue on HIV/AIDS, infectious diseases,
May 25th, 2023
The Legacy of a Disappointing HIV Clinical Trial — Does It Still Apply to HIV Today?
 A long, long time ago, back in the early exciting days of raltegravir, the first HIV integrase inhibitor, we learned something important from a clinical trial with disappointing results. The trial bore the (barely) hidden name of the company that developed the drug — SWITCHMRK, get it? — and had a profound impact on how we managed virologically suppressed patients for years.
A long, long time ago, back in the early exciting days of raltegravir, the first HIV integrase inhibitor, we learned something important from a clinical trial with disappointing results. The trial bore the (barely) hidden name of the company that developed the drug — SWITCHMRK, get it? — and had a profound impact on how we managed virologically suppressed patients for years.
What did we learn? Namely, that it was risky to switch stable people from their “high resistance barrier” regimen of lopinavir/ritonavir plus NRTIs to raltegravir plus NRTIs if they harbored viruses with NRTI resistance. Some of the participants who had a history of treatment failure who switched ended up experiencing virologic rebound with integrase inhibitor resistance, which made the switch to raltegravir not noninferior (sorry for the double negative) to continuing lopinavir/ritonavir.
The interpretation was that despite the potency and excellent tolerability of raltegravir — massively better than lopinavir/ritonavir — it wasn’t enough to maintain viral suppression reliably unless the NRTIs were also fully active. Based on these results, for years we steered clear of use of this valuable drug class in any setting where we couldn’t use at least one other fully active drug.
(My friend and colleague Joe Eron was the lead author on this study. I will note once again that Joe easily makes the top-5 in smarts in the HIV research world, no exaggeration, and that this was a very reasonable study design at the time, negative results notwithstanding. Unrelated, I wonder if Joe will attend this June 4 minor league baseball game in Charlotte, called Joe-Nanza, which aims to assemble the largest number of Joes at any single sporting event in history. It’s just a 2-hour drive from Chapel Hill!)
So now that we’ve reviewed SWITCHMRK, and a special event featuring Joes, let’s fast-forward to today, and contemplate a case. I’m sharing it with permission from Dr. Jezer Lezama, an ID doctor from Mexico who was looking for help (case details slightly edited):
PWH multi-drug resistance, w/high level R to PIs, NRTIs, NNRTIs, only etravirine partially active in 2013 but w/o previous exposure to integrase inhibitors. Rescue Tx: DRV600/r BID + RAL + ETV with viral suppression since then. Simplify? To which regimen?
He provided the 2013 resistance genotype, and yes, this is a challenging virus:
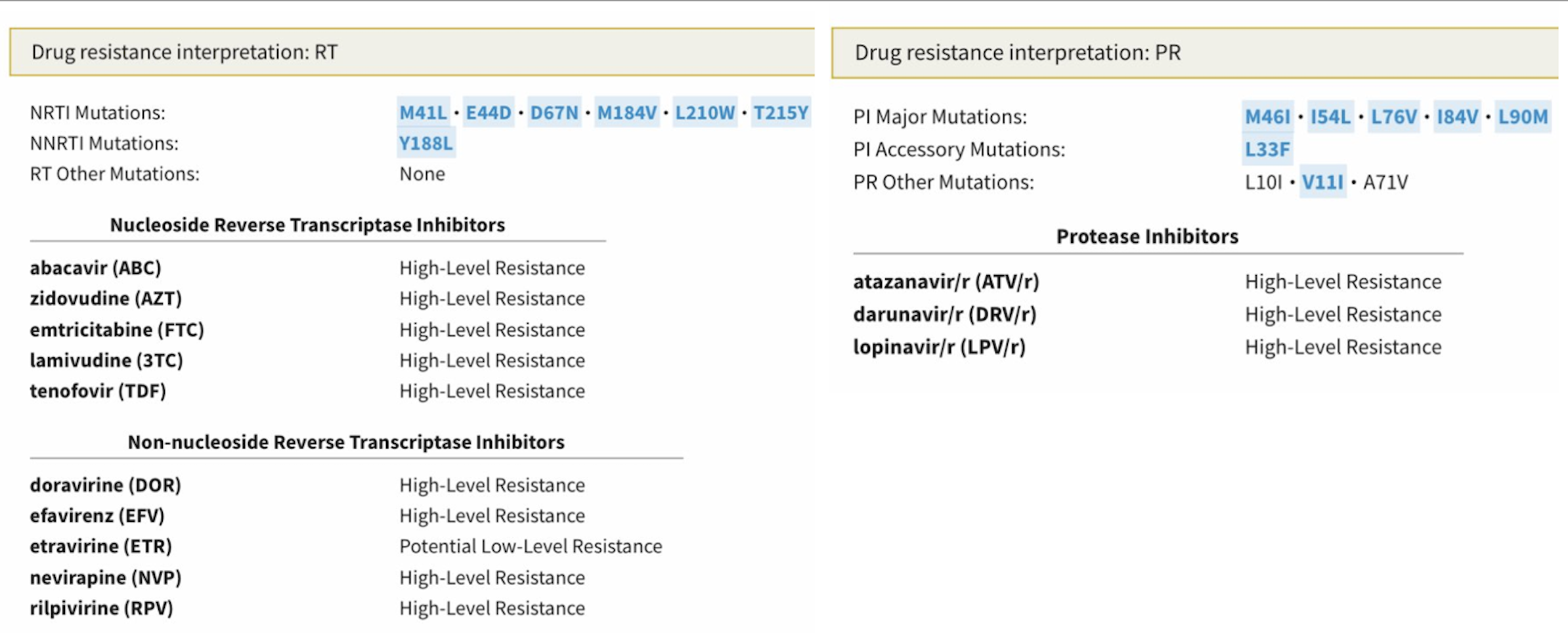
There’s a lot there to digest, so allow me to provide a quick interpretation, sparing you the trip to the Stanford HIV Drug Resistance Database:
- NRTIs: The virus has the “TAM-1” pathway (M41L, L210W, T215Y), plus another thymidine-associated mutation (D67N), so even tenofovir activity is reduced. M184V, the magical 3TC/FTC mutation, partially reverses this tenofovir resistance.
- NNRTIs: For this drug class, we find the important Y188L mutation, which confers high-level phenotypic resistance to all NNRTIs except etravirine. Trivia buffs will recall that Y188L is present on HIV-2 isolates, which is why NNRTIs don’t work against HIV-2.
- PIs: Not much to like here — there are 3 major darunavir-associated mutations (I54L, L76V, I84V), two minor darunavir mutations (L33F, V11I), plus a couple of other major PI mutations (M46I, L90M). I bet there’s some fosamprenavir treatment failure in the past. If this person had a phenotype done, I suspect the fold-change loss of susceptibility would suggest that the darunavir isn’t doing much in this regimen, twice-daily dosing notwithstanding.
- INSTI: Not done, but presumably no resistance.
Fortunately, the brief history does give us good news about how he’s doing clinically — virologic suppression since 2013 on the “TRIO” regimen of twice-daily darunavir, twice-daily raltegravir, and twice-daily etravirine. As I’ve mentioned before, I cannot begin to describe how transformational HIV drug development was in the late 2000s, when we suddenly had these three new tools (especially raltegravir) for our highly-adherent PWH with multidrug resistance.
Some experienced an undetectable viral load for the very first time in their lives. It was truly a cause for celebration. Yay!
Dr. Lezama’s specific question now is — can he simplify the treatment to bictegravir/emtricitabine/tenofovir AF? Why or why not?
What do you think? Next week we’ll review the results.


Doravirine+dolutegravir daily
Bic/tAF/FTC with DRVr
This will simplify it, provide an integrase but also maintain some activity from darunavir.
I would not have trusted elvitegravir/TAF or TDF based in the presence of M184V as I have seen many cases of emergence of elvitegravir resistance.
however have not seen failures of BIC/FTC/TAF in the presence of M184V!
although I’d prefer to combine BIC/FTC/TAF plus doravarine in this case for “peace of mind” ! 🙂
Stop the BID Darunavir and start fostemsavir( decreased pill burden) + dovato. Stop raltegravir and etravirine
Considering bictegravir similar efficacy to dolutegravir, would consider switch reasonable safe to BIC/TAF/F based of NADIA and VISEND data suggesting high levels of suppression in patients with ACTIVE viremia and pan NRTI resistance. Patient is already suppressed and can monitor a bit more closely initially for changes in VL. Pill saving and eliminations of DDIs is significant
I think Biktarvy will work. For the anxious physician – you can add Lenacapavir once every 6m
If available in Mexico, I’d suggest a lenacapavir-containing regimen. For example, LEN+BIC/TAF/FTC
Maybe using the bic/taf/FTC with something else. Keeping darunavir would be an option, despite de pill burden and DDI. Fostemsavir or even Maraviroc would be an option if the patient has CCR5 tropism ( I don’t know if fostemsavir is available in Mexico). Using just BIC/TAF/FTC or FTC/TDF/DOL would be an option, but close monitoring is necessary
No doubt that BIC/TAF/F would work just fine in this case The results of the SECOND-LINE study 10 years ago and more recently NADIA showed that you could recycle nukes even in the presence of ‘high level resistance’, add a fully active ARV from another drug class and Robert is your mother’s brother (as they say)!
Suspect this person has more extensive resistance than most/all people in NADIA and at 96 weeks there were failures with resistance to DTG.
They may well be fine on BIC/TAF/FTC but is it worth the risk given the cost of failure? Also you’d need to cover the switch with extras given ETR’s induction effects and this may be a disaster as the ETR is crucial in this case so stopping and relying on sub-therapeutic BIC without any help is mad.
If desperate to simplify why not DTG/3TC + ETR? Two active agents and 3TC thrown in for good measure. You’d have to dose the DTG BD so unclear how much this simplifies things but gets them off the PI.
Dolutegravir + Doravirine
Oof, tough situation. Might be the first time in a while that I would get a phenotype, I think the question of how much utility we have from PIs is a good question. I’m pretty liberal with using DRV/c over DRV/r BID, but this is definitely a BID or bust situation.
The ETR somewhat cancels the /r or /c boosting, so I always try to get people out of this scenario. Once had a patient on DRV/r BID + ETR + Methadone, it is like a 3 drug mexican standoff. Depending on which drug you miss, you overdose or you go into withdrawal!
I like all the comments on going for an INSTI based STR + LEN. If you would be game for DOR+DTG, why not just use CAB/RPV/LEN? I think that is a little risky and the last thing we need here is to add INSTI resistance.
I’d keep the current regimen, if viremia in the future, I’d check a phenotype and if PIs still have utility, then do BIC/TAF/FTC + DRV/c + LEN.
bictegravir/emtricitabine/tenofovir AF might work, but would feel better with something else, such as leaving in the etravirine, though that would still be a twice daily regimen
As some others have commented, maraviroc, fostremsavir or lenacapavir would be other drugs that could be added, but might be nice to save these for the potential next salvage regimen
Injectable Carbotegravir + Rilpivirine can be an option