An ongoing dialogue on HIV/AIDS, infectious diseases,
July 19th, 2017
Mystifying Cochrane Library Review on HCV Therapy Elicits Strong Response from IDSA
 Last month, the Cochrane Review published a controversial paper on HCV therapy that left many ID doctors and hepatologists perplexed.
Last month, the Cochrane Review published a controversial paper on HCV therapy that left many ID doctors and hepatologists perplexed.
After reviewing 138 randomized clinical trials using directly acting, non-interferon based therapies, they came to the following conclusions:
- The use of sustained virologic response (“SVR”) — or “cure”, if you want to use plain English — as a valid endpoint for predicting clinical outcomes is questionable.
- There is currently insufficient evidence that treatment with DAA-based regimens improves clinical outcome.
- The studies reviewed were at high risk of bias, so tended to overestimate benefits and minimize harm.
- More randomized clinical trials are needed.
Anyone — clinician, researcher, or patient — who has experienced the miraculous advances in HCV therapy that started in 2014 could easily be scratching their heads at these conclusions.
The FDA might be surprised as well, since they have allowed SVR as an appropriate “surrogate” marker of the effectiveness of HCV therapy for some time.
Fortunately, we now have a focused, persuasive response by the IDSA, just published in Clinical Infectious Diseases.
I strongly encourage anyone who doubts the clinical benefits of curing HCV to read the full paper, but in essence the argument goes like this:
- The review was overly selective in the papers it included. Remember, many HCV trials could not include a control group since DAA therapies were so rapidly effective and well tolerated it would have been unethical. These non-controlled studies were not included in the review.
- HCV cure as an appropriate marker for treatment efficacy was established during the years of interferon-based therapy. Liver inflammation (as measured by biopsy or serial LFTs), fibrosis, portal hypertension, splenomegaly, even cirrhosis improved in those with SVR. And I would add that some surrogate markers are more intuitively obvious than others — and you can’t really get more obvious than curing the very infection that’s causing the disease. HCV RNA is not an obscure, indirect tumor marker (oncology), or a change in lipids (cardiology). It’s analogous to HIV RNA in HIV therapy, only better. And is there any plausible biologic reason why HCV cure with DAAs might be less effective in improving clinical outcomes than using interferon?
- The time horizon to see the full clinical benefit for HCV cure will take many years. We’ve only had these therapies widely available since 2015 — hardly enough time to see reductions in the incidence of long-term complications such as cirrhosis or hepatocellular carcinoma. Note that we’ve already seen benefits in HCV transmission from treatment in a clinical cohort of MSM from Europe.
- Despite this short time period of DAA availability, clinical benefits have already been observed with HCV cure. These include resolution of vasculitis, spontaneous remission of non-Hodgkin lymphoma, and — perhaps most remarkably — stabilization or improvement in those with the most advanced forms of HCV liver disease.
I will note that this isn’t the first time a “systematic review” of an Infectious Disease treatment under the Cochrane name ended up with a surprising conclusion.
Remember this one on HIV treatment with TDF/FTC/efavirenz? The one which stated there was insufficient evidence to support its use, despite numerous randomized clinical trials documenting its efficacy? And its widespread adoption in clinical guidelines?
It may be hard to find today, since it was later withdrawn.
Save
July 9th, 2017
Should You Answer Medical Questions from Clinicians You Don’t Know About Patients You’ve Never Seen?
 This email popped into my inbox the other day from a person I’ve never met:
This email popped into my inbox the other day from a person I’ve never met:
Hi Dr. Sax,
I do mostly hospital-based ID in Pennsylvania, and was consulted on a newly diagnosed HIV patient with CD4 10, viral load 210,000, and lymphoma. I started him on Truvada and dolutegravir, which is going well so far. Because he complained of blurred vision, he had an ophtho evaluation yesterday which showed CMV retinitis. My drug-interaction checker says I can’t use valganciclovir with either tenofovir or abacavir, and if I replace the Truvada with a boosted PI, it will interact with his chemotherapy. What should I do for his ART?
Thanks so much.
Marie
There are two issues with this email worth discussing.
The easy part first — the medical question. Here’s my response:
Hi Marie,
There is no significant interaction between ganciclovir and tenofovir alafenamide, and even the interaction with tenofovir DF is theoretical, not an absolute contraindication. No interaction with abacavir either, so not sure where you are getting your information! (Use this site, it’s awesome: www.hiv-druginteractions.org.) So switch the Truvada to Descovy (tenofovir alafenamide/emtricitabine), that’s all you need to do. Safer for kidneys and bones, too.
Regards,
Paul
The second item to cover is whether we should be answering questions like this at all. Remember, this is from a person I don’t know, asking about a patient I’ve never seen.
Though I obviously responded to the query, there are a few reasons not to answer questions from clinicians you’ve never met about patients you haven’t seen.
The medical information might not be correct, or complete enough, to make a good recommendation. If you make the wrong suggestion, or your recommendation is misquoted, there’s the potential for patient harm. Even worse: if your name is in the chart, there’s a medicolegal risk — especially if you review patient data sent to you. The risk may be small, but who wants to take that chance?
And if you ask an economist, they would say it definitely makes no sense to answer these questions — not only are you being paid nothing, but there’s little chance of downstream revenues, and it takes time away from other remunerative tasks and opportunities.
But economists can be short-sighted, and this is one of those times. Obviously I thought it was better to answer the question than to ignore it for a bunch of reasons.
- Answering helps the patient. Sometimes cliches are true: helping people remains the primary reason most of us went to medical school to begin with.
- Answering helps the clinician. When I see a difficult case of coccidioidomycosis, I of course call an expert in this tricky fungal infection; cases of cocci are rare in Boston. And I’m so grateful when John Galgiani responds, given his voluminous experience. Ditto various cases over the years involving rapidly growing mycobacteria (Richard Wallace), bartonella (Jane Koehler), toxoplasmosis (Jose Montoya), Mycobacterium avium complex (Chuck Daley, Gwen Huitt), cytomegalovirus (Richard Whitley), and many others. Thank you!
- It was a straightforward, focused question, presented clearly. I didn’t quote the whole email, which included numerous other details about the chemotherapy regimen, but those were thoughtfully placed at the bottom of the communication.
- The person asking was polite. No dreaded Red Exclamation Point indicating that this was of the utmost urgency. (Here’s a thought — let’s ban that particular means of communication.) No “Thanks in advance for your rapid reply.” (Ugh.)
- It’s flattering when someone asks you questions in your area of expertise. Gosh, Marie chose to ask me about her patient’s HIV therapy? When there are so many other people she could have asked? Hey, maybe I should be thanking her! (Of course she might have sent the same email to 20 others, but … who’s to know?)
The bottom line is that I think we should be helping out other clinicians when we can — it’s just the right thing to do.
Save
July 2nd, 2017
Delafloxacin, a New Quinolone, Is Approved for Skin Infections — But That’s Not Where It’s Really Needed
The history of the fluoroquinolone antibiotics can be divided into four eras, alternating good news and bad:
- Ciprofloxacin is approved — it covers everything, and is miraculous. We’re talking some tough customers here. Pseudomonas aeruginosa! Staphylococcus aureus! Neisseria gonorrhoeae! Plus, pretty much every gram negative causing urinary tract infections. There was no intravenous formulation initially, but that hardly mattered since it had great oral absorption. I remember as a resident seeing a patient with a polymicrobial diabetic foot infection in 1990 who was facing a long course of IV antibiotics — but instead went on ciprofloxacin orally at the suggestion of a brilliant ID consultant. As I said, miraculous.
- Resistance to quinolones emerges — and quickly. Staph aureus, especially MRSA, quickly became resistant to quinolones. Then Pseudomonas aeruginosa. Then a host of other gram negatives urinary pathogens. Then gonorrhea. Then enteric infections. Plus, we learned ciprofloxacin never should have been approved for treatment of pneumonia to begin with — whether it was problems with poor pneumococcal activity, or inadequate lung penetration, or both, it clearly was a bad respiratory tract drug. Oh well, it was fun while it lasted!
- Respiratory fluoroquinolones ride to the rescue. Levofloxacin, moxifloxacin, and especially trovafloxacin picked up many of the bugs that ciprofloxacin was missing. In case those weren’t enough options, there was sparfloxacin and grepafloxacin and gatifloxacin and gemifloxacin too. All had far greater gram positive coverage than ciprofloxacin, especially for streptococcal isolates. Moxifloxacin and trovafloxacin also covered anaerobes. Trovafloxacin was FDA approved for no fewer than 14 indications — a world record! And while many predicted inevitable pneumococcal resistance with the extraordinarily widespread use of these drugs, it never became that much of a problem. There was a rule on most medical services that every patient had to receive at least one dose of levofloxacin before discharge. (I made that up.)
- TOXICITY. All caps, italicized, and bolded, for a reason. Quinolones, it turns out, are not so safe after all. The FDA pulled trovafloxacin (hepatotoxicity), grepafloxacin (QT prolongation), sparfloxacin (photosensitivity), temafloxacin (hemolytic anemia and allergies) and gatifloxacin (hypoglycemia) from the market for safety concerns. The few surviving quinolones have the dreaded black box warning for serious adverse effects. This describes not only idiopathic tendon rupture, but also “disabling and potentially permanent serious side effects that … involve the tendons, muscles, joints, nerves, and central nervous system.” While in some patients these side effects are difficult to distinguish from the multitude of other causes of fatigue, poor concentration, and joint pain, there’s little doubt that quinolones are highly toxic to certain individuals. Potentially life-threatening QT prolongation and Clostridium difficile — two problems separate from the quinolone toxicity syndrome, but still serious — can be added to the mix. The toxicity profile is bad enough that the FDA advised, in 2016, to limit outpatient prescribing of quinolones to “those who do not have alternative treatment options,” a major action for this regulatory board.
Into this messy mix, and arguably against great odds, we now have a new fluoroquinolone — delafloxacin. It’s available in oral and intravenous formulations (both given twice daily), is FDA-approved for treatment of skin and soft-tissue infections (based on the results of this study), and most notably, has activity against both Pseudomonas aeruginosa and Staph aureus, including MRSA.
In vitro, its coverage also includes most coagulase negative staphylococci, enteric gram negative rods, respiratory pathogens, Neisseria gonorrhoeae, Bacteroides fragilis, and Mycobacterium tuberculosis.
Various reviews (here’s a good one) will cite the fact that unlike most quinolones, which are zwitterionic, delafloxacin is anionic, leading to increased accumulation in bacteria. That certainly sounds impressive, and a quinolone with reliable anti-pseudomonal and MRSA coverage currently does not exist.
However, coverage and biochemistry notwithstanding, we might wonder why we need another treatment for skin infections, especially with the toxicity profile of quinolones. Indeed, the FDA-approved medication guide for the drug goes to great lengths to warn people about potential side effects.
There are two answers to this mystery. First, it’s easier to get FDA-approval for treatment of skin and soft-tissue infections than it is for other indications. Cue up your favorite low hanging fruit analogy.
Second, the drug was given priority review by the FDA since it was designated as a Qualified Infectious Disease Product (QIDP) under the Generating Antibiotic Incentives Now (GAIN) Act of 2012. While this act encourages novel antibiotic drug development, these approvals can leave clinicians scratching their head about why the drug is available at all:
Oh joy. Another MRSA skin drug. Will wonders never cease. I've had to update my table. Can anyone spot the trend? pic.twitter.com/td4BPCFgAS
— Brad Spellberg (@BradSpellberg) June 28, 2017
Ever the optimist, I’m hopeful that now that delafloxacin is approved, we will eventually see studies documenting its efficacy in clinical settings of greater unmet need.
Based on this search, it looks like trials in community-acquired pneumonia (ho-hum) and gonorrhea (good) are in the works. (The gonorrhea study was stopped — see comment below.)
Here are a few more study ideas, with admittedly much tougher patient populations and study endpoints, but leveraging delafloxacin’s antibacterial spectrum, bactericidal activity, and excellent oral bioavailability:
- A randomized, phase 3 clinical trial comparing oral delafloxacin with intravenous vancomycin or daptomycin for Staph aureus bacteremia. Randomization would occur after clearance of blood cultures. Stratify based on MRSA vs MSSA.
- A randomized, phase 3 clinical trial comparing oral delafloxacin with intravenous therapy in treatment of spinal osteomyelitis due to susceptible organisms. Randomization after stable on IV therapy. Stratify based on risk factor (injection drug use vs other) and causative organism (MRSA vs other).
- A randomized, phase 3 clinical trial comparing oral delafloxacin with intravenous therapy in diabetic foot infections. Stratify based on whether the study subjects underwent surgical debridement.
- A randomized, phase 3 clinical trial comparing oral delafloxacin with intravenous ertapenem or cefepime for treatment of complicated urinary tract infections due to resistant gram-negative organisms. Stratify based on Pseudomonas aeruginosa vs other.
So that’s 4 tough areas of ID practice to start. But why stop there? How about looking at delafloxacin in studies of MDR tuberculosis? Or non-tuberculous mycobacteria? Many interesting possibilities.
Ready to enroll?
And though totally unrelated, this made me laugh:
[youtube https://www.youtube.com/watch?v=0nkgw-4VB0M]
June 18th, 2017
On Father’s Day, A Rumination on Families with Lots of Doctors
So was my father’s father. And my father’s uncle. And my father’s cousin.
But that’s not all. My father’s brother was also a doctor — he loved being a doctor more than anyone on the planet, and attended neurology meetings long after he retired, right up until the time he died last year.
My father’s brother-in-law (i.e., his sister’s husband) — you guessed it, a doctor.
It gets even more ridiculous. I have two first cousins who are doctors, and three first cousins married to doctors.
And I, of course, am married to a doctor myself. Her brother? A doctor.
Having all of these MDs readily available has had quite an influence on family dynamics. At one of our gatherings, my brother — not a doctor, he’s in finance — told an elderly aunt the name of the bank he was working for at the time.
Hard of hearing, she responded, “What medical school?”
These gatherings, not surprisingly, can sometimes seem more like medical grand rounds than holiday celebrations. You almost expect someone at Thanksgiving Dinner to say, “May I have the first slide, please?”
The medical profession runs so strongly in my family that at times I suspect our various dogs are, in their own doggie world, the dog-equivalent of doctors. A bit nerdy, scholarly, caring for others.
So why is it that some families take to medicine so avidly? It certainly isn’t a universal phenomenon — I routinely ask residency and fellowship applicants if there are doctors in their family, and most of them say no.
You can try researching this question, but it’s a tricky thing to search. (I tried.)
Let’s keep it simple, and list the reasons why people become doctors to begin with:
- You help people. We never really need to ask the existential question, so why am I doing this job again?
- It’s interesting. Patients, colleagues, scientific discoveries, technical challenges, policy issues — medicine is endlessly fascinating. No good doctor feels he or she has mastered their field; learning all the time is fun.
- You have a steady income. No, doctor salaries won’t touch hedge fund managers or real estate developers, or approach the revenues you might get when you sell your high tech startup to Google. And you won’t be able to afford the premiere real estate in the Bay Area or Manhattan — but let’s face it, nobody’s poor here.
- It’s prestigious. People like doctors. We might not be as popular as we once were, but it sure beats the reputation of lawyers, or politicians, or the CEO of Uber.
- You can do a lot of different things as a doctor. Aside from my wife (pediatrician) and me (ID doc), included in my family collection of doctors is a psychiatrist, a maxillofacial surgeon, an emergency room doctor, a sleep specialist, an obstetrician-gynecologist, a general internist, a nephrologist, and a Professor of the History of Medicine. I can assure you none of them does the same thing in a typical work day — yet all are doctors.
If those are the reasons why people choose medicine as a career, it still doesn’t quite explain why some families — like mine — have so many doctors.
Maybe we just suffer from a lack of imagination.
Happy Father’s Day! And yes, Dad, going to medical school was the right decision after all.
June 10th, 2017
What’s Your Favorite Antibiotic? A Fantasy Draft
 Over on the journal Open Forum Infectious Diseases (that’s “O-F-I-D”, not “Oh-FID”), the generous people from IDSA and Oxford University Press have allowed me to record a series of podcasts, interviewing various interesting people in the ID field.
Over on the journal Open Forum Infectious Diseases (that’s “O-F-I-D”, not “Oh-FID”), the generous people from IDSA and Oxford University Press have allowed me to record a series of podcasts, interviewing various interesting people in the ID field.
This time, however, I strayed from the usual format and asked my colleague Rebeca Plank to join me in a “draft” of our 5 favorite antibiotics.
Which is emphatically not to imply that Rebeca isn’t interesting. On the contrary — she happens to have one of the most impressive pen collections in all of Eastern Massachusetts.
Still, you may wonder, why did we do this? Several reasons:
- We sensed a burning need for this this critical educational resource, which as you will see teaches fundamental truths about these important therapeutic tools.
- One of us wanted to honor the upcoming baseball draft (hint: Rebeca couldn’t care less about baseball).
- Someone gave me a USB microphone, and it was sitting around doing nothing for way too long.
In short, mostly we did it for fun.
Take a listen! And while you’re at it, two questions:
- What are your favorite antibiotics, and why?
- What should we draft next?
Hope you enjoy.
(H/T to Joe Posnanski and Michael Schur for the draft idea.)
June 4th, 2017
Can’t HIV Serodiscordant Couples Now Just Have Children the Regular Way?
MMWR just published a paper entitled, Strategies for Preventing HIV Infection Among HIV-Uninfected Women Attempting Conception with HIV-Infected Men — United States, and it’s both a welcome and a very strange document indeed.
It’s welcome because it acknowledges that serodiscordant couples may wish to have children without the use of an HIV-negative sperm donor. Advances in HIV prevention mean they can drop their categorical recommendation against insemination with semen from HIV-infected men, one they originally made in 1990.
But it’s strange because, right alongside treatment of the HIV-positive man with antiretroviral therapy (ART) and pre-exposure prophylaxis (PrEP) for the woman, is a fairly lengthy data summary on “sperm washing”, a strategy many would argue has outlived its usefulness.
This is the short version of the procedure:
Another strategy that can be used in conjunction with HAART and PrEP is collection and washing of the male partner’s sperm to remove cells infected with HIV, followed by testing to confirm the absence of HIV prior to intrauterine insemination (IUI) of the female partner or in vitro fertilization (IVF).
Does one really need to put couples through this expensive and time-consuming process when the risk of transmission is unmeasurably tiny, if not zero, if the man is on suppressive ART and the woman is taking PrEP? Can you imagine the number needed to treat to prevent one additional case of HIV transmission with sperm washing in addition to ART and PrEP?
It’s like wearing a belt, suspenders, and using duct tape to keep your pants up (which is probably not the best analogy when discussing something that has to do with sex, but I’m going with it).
As a quick reminder:
- HPTN 052: Zero transmissions from infected partner if HIV RNA suppressed.
- PARTNERS Study: Zero transmissions from infected partner if HIV RNA suppressed — this while the couples were practicing “condomless sex” (otherwise they couldn’t be part of the study).
- PrEP Studies: Among adherent participants, > 90% efficacy despite high risk behavior or high community risk.
Now we don’t want to tempt the Gods of Never Say Zero with hubris about this unmeasurably low risk, but I clearly wasn’t the only one who found the paper’s emphasis on sperm washing peculiar.
Here’s a take from Ben Young, Senior Vice President/Chief Medical Officer of the International Association of Providers of AIDS Care:
https://twitter.com/benyoungmd/status/870675504004648960
(“U=U” stands for “undetectable equals uninfectious”.)
Just for fun, I thought I’d check in with Pietro Vernazza, architect of the 2008 controversial “Swiss Statement” that has, for the record, turned out to be 100% correct.
Here was his response:
I was shocked about the report. In Switzerland, we have not seen any new infections among couples trying to conceive without using any additional safeguards (aside from treatment of the infected partner). The Swiss statement is very widely accepted.
And then, because what the hell, we deserve it, he added this:
But what should we say? We also don’t understand how a country can withdraw from the Paris agreement, or try to build a wall to Mexico, or to withdraw health insurance for their citizens.
Hey, I’m a huge fan of our CDC, this odd report notwithstanding. But on these issues, you’ll get no defense from me!
And just curious — is there anyone out there still strongly advocating sperm washing for serodiscordant couples wanting to have children?
Save
May 29th, 2017
Healthcare Providers Shouldn’t Come to Work While Sick, but They Do — Here’s Why
Let’s start with two questions:
- Have you ever seen a doctor, nurse, PA, pharmacist or other person directly involved in patient care wearing a surgical mask because they have a respiratory tract infection?
- Has this mask-wearing person ever been you?
Bold prediction: Virtually every reader who works in a hospital or large office practice answered “Yes” to #1. Some of you might even have said “Yes” to #2.
Clearly healthcare providers do go to work while sick, and the mask-wearing is our way of saying, “I care.”
But if we really cared, shouldn’t we just stay home?
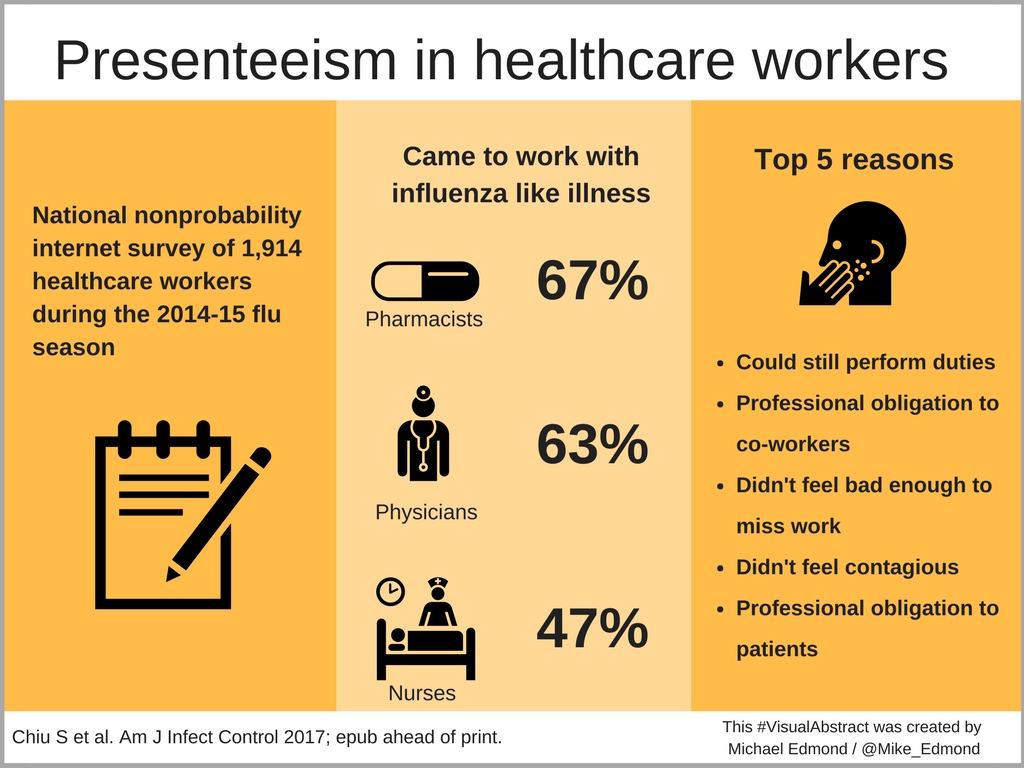
Of course we should stay home. But we don’t. Here’s a recent paper on the topic highlighted by Mike Edmond on his excellent blog, using the nifty “visual abstract” method to present the results:
Remember, surveys aren’t exactly perfect in eliciting whether people are doing some ill-advised behavior. People lie, even in anonymous polls. I know, shocking.
So consider the above estimates of this “presenteeism” a minimum. Yikes. That’s a lot of sniffling, coughing, and potentially mask-wearing healthcare providers out there.
So let’s examine the top 5 reasons why people got to work with an influenza-like illness in a bit more detail; I’ll play the part of the person choosing each response:
- Could still perform duties. Hey, does having a cold preclude me from typing and clicking boxes? That’s mostly what I get paid to do these days, right?
- Professional obligation to co-workers. Who else will do my work? And how can I ask for coverage for what might be “just a cold”?
- Didn’t feel bad enough to miss work. I told you I could type.
- Didn’t feel contagious. If I work while having a cold, I promise to double-down on the hand hygiene — you can call me “Dr. Purell” — and (though it’s embarrassing) to wear a mask. And no one really knows how long a cold is contagious. Symptoms can easily last 1-2 weeks, should I be out the entire time? Impossible.
- Professional obligation to patients. My patients will be mad if I don’t show up. They would prefer to see a sniffly, red-eyed, coughing me than a covering clinician, or to wait a week until I’ve recovered. And obviously no one can provide the brilliant, compassionate, and efficient care that I do.
Jokes aside, presenteeism gives infection control practitioners a major challenge. It’s even more intractable when you factor in the most common reason cited by healthcare providers at long-term care facilities, which is the inability to afford lost pay.
One practical problem we could work on is directly related to reason #4, “Didn’t feel contagious.”
While it’s intuitively obvious that a healthcare worker with pulmonary tuberculosis, active salmonellosis, or highly symptomatic influenza shouldn’t be working, what about milder illnesses that can, in certain hosts, be life-threatening? Thinking about you, RSV, adenovirus, and parainfluenza virus.
Perhaps it’s time we all got a multiplex PCR machine for home use. It would look great in the living room.
Save
May 21st, 2017
The Curious Case of M184V, Part 1
 Thanks to our sophisticated research team here at NEJM Journal Watch, we have an excellent idea who reads this thing for its scintillating ID/HIV content.
Thanks to our sophisticated research team here at NEJM Journal Watch, we have an excellent idea who reads this thing for its scintillating ID/HIV content.
Most of you are clinicians — doctors, nurses, PAs, PharmDs. A smaller proportion are researchers, lab-oriented types who wandered over here unexpectedly after an errant search, expecting the latest in CRISPR-Cas9 gene editing and instead getting an ID Link-o-Rama, a rumination on vintage medical photos, and a mysteriosis about listeriosis.
But another divider is whether you consider yourself an HIV specialist or not. A grab bag of ID (mostly), primary care, and other subspecialty clinicians, HIV specialists know and ruminate over lots of the same stuff even though there’s no formally designated HIV specialty by the American Board of Internal Medicine.
And today’s topic is most definitely an HIV-focused one, triggered by an email I received last week from one of my colleagues:
Subject: M184V
Paul,
What’s your go-to regimen in the setting of a solo M184V?
Jon
For the non-HIV specialist readers, allow me to decipher the code in the above question. “M184V” is the shorthand for methionine replacing valine at position 184 in reverse transcriptase. It is by far the most commonly encountered nucleoside reverse transcriptase inhibitor (NRTI) mutation after failure with regimens containing lamivudine (3TC) or emtricitabine (FTC).
And with that single paragraph, I’ve hinted why HIV drug resistance — and genotypes in particular — baffle some of even the most astute and brilliant ID clinicians. It’s like reading about the coagulation cascade or the complement system. You have to work with this stuff frequently to understand the lingo.
But for those seeing HIV patients on a regular basis (especially as outpatients), this question — what should be done after M184V? — is both quite relevant clinically and, surprisingly, not readily answerable from the literature.
Remember, M184V is a special mutation — it does some very weird things:
- Viruses that harbor M184V don’t replicate well. In virology parlance, they’re “less fit.”
- M184V causes marked phenotypic resistance to 3TC/FTC, but this doesn’t translate clinically. Or, to cite the invaluable Stanford HIV Drug Resistance Database, “M184V/I are selected by 3TC/FTC and reduce susceptibility to these drugs >100-fold.” For an analogy, think of high-level gentamicin resistance in an enterococcus isolate — but then ignore it and use gentamicin anyway. Because unlike these enterococci, where gentamicin would be useless, studies show that 3TC still exerts significant antiviral activity despite this loss of phenotypic activity. I’ve cited these studies before, but they deserve emphasis: Virologic rebound occurred after stopping 3TC in patients who already had developed M184V; and 3TC alone slowed CD4 decline more than no treatment despite M184V being present in all patients.
- M184V influences the in vitro susceptibility of certain other NRTIs in a favorable way. Yes, with M184V, susceptibility to tenofovir, zidovudine, and stavudine improves. In other words, an M184V containing virus is more susceptible to tenofovir than a wild-type virus, a phenomenon referred to as hypersusceptibility. Someone much smarter than I can explain the molecular mechanism of this phenomenon (it certainly won’t be me).
Because of these odd effects, and because both 3TC and FTC are so well tolerated, there’s a practice (not universally observed) of continuing 3TC or FTC even after M184V has been selected. But should this be done?
And with that background, let’s get back to the question in Jon’s email — what to do after M184V?
Imagine this case — a patient who failed a regimen of dual NRTIs plus an NNRTI (let’s say TDF/FTC/EFV) some time in the past. He had a genotype then showing M184V and K103N (conferring resistance to efavirenz), and then was lost to follow-up for a few years.
He now shows up saying he wants to start treatment again. Let’s give him a CD4 cell count of 250 and a viral load of 50,000. He of course wants as few side effects, and as few pills, as possible.
What would you choose as his antiretroviral regimen?
May 14th, 2017
Poll: Which Feature of Electronic Health Records is Most Important to Patient Care?
 The first electronic medical record I used regularly — called “BICS” — initially had one purpose. It was a tool to look up a patient’s lab results.
The first electronic medical record I used regularly — called “BICS” — initially had one purpose. It was a tool to look up a patient’s lab results.
Simple, reliable, and blazingly fast, it did one thing remarkably well.
Later, one of our Emergency Department doctors, who happens to have impressive coding skills, worked with a team to add a simple ambulatory medical record (medications, allergies, a problem list, progress notes).
Soon after, they released an astonishingly efficient inpatient order entry system, one that relied on keyboard shortcuts. Use of the mouse was definitely for amateurs.
After a brief learning period that took the interns around a nanosecond, all the clinicians loved it. It was so popular that the medical housestaff even held a party in its honor, something they called “OlymBICS,” with teams competing to see who could enter orders the fastest.
Much of its success, I’d argue, had to do with the simplicity — it didn’t overreach. Today, of course, all the big EHRs (or EMRs, or whatever you want to call them) try to do all things for all people — doctors, nurses, patients, administrative staff. I wouldn’t be surprised if there are features for the hospital cafeteria and catering services.
And because the range of these activities is so broad, EHRs have become bloated, complex, and inefficient. The complexity steals attention away from our patients as we try to satisfy the insatiable screen, keyboard, and mouse.
There’s just so much to do (and so many opportunities to do it wrong), it’s hard to concentrate.
In a piece called “Death By A Thousand Clicks,” some local colleagues write the following:
It happens every day, in exam rooms across the country, something that would have been unthinkable 20 years ago: Doctors and nurses turn away from their patients and focus their attention elsewhere — on their computer screens … EMRs have become the bane of doctors and nurses everywhere. They are the medical equivalent of texting while driving, sucking the soul out of the practice of medicine while failing to improve care.
Yes, we all recognize that feeling!
These problems notwithstanding, it’s clear that not even the crankiest EHR critic would propose that we go back to the days of paper charts, radiology film libraries, or having to call the lab to get patient test results.
Part of what makes the problems with EHRs so frustrating is that there is so much potential for excellent, intuitive, and interoperative systems. EHRs already do some things very well indeed.
The British struggled when their EHRs went down in the recent WannaCry cyberattack, and not just because they were still using an unpatched version of Windows XP.
(XP? Seriously? Yikes.)
So to detail the EHR benefits, and in honor of BICS (may it R.I.P.), I list below several widely available EMR functions.
You, dear reader, will choose the one feature you would miss the most during patient care if it suddenly were no longer available. In the comments section, feel free to elaborate why you chose what you did.
And I’m pretty sure I can predict the loser of this poll.
Save
May 7th, 2017
CRISPR and HIV “Cure,” Zinc for Colds, New AIDSInfo Site, CROI Dates, Vanco Pricing, and More: I Can’t Believe It’s May ID Link-o-Rama
 A few Infectious Diseases/HIV items to consider as we wait (and keep waiting!) for the warm weather to arrive in chilly, and often wet, New England:
A few Infectious Diseases/HIV items to consider as we wait (and keep waiting!) for the warm weather to arrive in chilly, and often wet, New England:
- CRISPR-Cas9 excises latent HIV from the cells of humanized mice. With the usual caveat about not overreacting (like this) to an animal model, this CRISPR strategy seems like a more viable HIV cure intervention than other approaches. Nonetheless, we still have a long way to go before something like this is tested in humans and deemed to be safe.
- Zinc might shorten the duration of cold symptoms after all. Time for a fully powered, randomized, double-blind trial!
- Do the rare late relapses after HCV cure warrant checking HCV RNA a year after completing therapy, and not just at week 12? These guidelines from the American Gastroenterological Association Institute state that “some clinicians may think it prudent.” (Read that with a George Bush Sr. accent, via Dana Carvey.) Probably the best reason to re-check HCV RNA is to document re-infection, not a relapse. Regardless, this strategy does make sense.
- Largest measles outbreak in Minnesota in decades sickens children, leads to hospitalizations. (Edit: Excellent original coverage here, including comments from discredited vaccine researcher Andrew Wakefield, who states, “I don’t feel responsible at all” for the outbreak. Good grief.) And another outbreak in Portugal led to the death of a 17-year-old girl. Wouldn’t it be great if we had a simple, effective way of preventing measles? Oh, wait.
- The AIDSinfo site has been extensively updated. Well worth checking out for all your guidelines, patient information, and educational needs. Mobile apps, too. One pet peeve — I prefer “ART” over “ARV” as an abbreviation for “antiretroviral therapy.” How about you? Let’s do a poll:
Quick poll:
What abbreviation do you prefer for "antiretroviral therapy"?
— Paul Sax (@PaulSaxMD) May 7, 2017
- Tick-related illnesses could be a particular problem this year. Mice + Acorns = potential explosion in tick population. Get that doxycycline ready.
- One penicillin dose for early syphilis in people with HIV is good as three. More is not always better. And isn’t it extraordinary that Treponema pallidum still hasn’t figured out how to develop resistance to penicillin?
- This superb review of the book Drug Dealer, M.D skillfully outlines how we got into this opiate addiction mess. I kept nodding with agreement while reading this piece by my friend and colleague Abbie Zuger. I think it should be required reading for all current doctors, nurses, PAs, PharmDs, as well as students of these healthcare professions.
- The Massachusetts Medical Society voted to support medically supervised safe injection sites. There is excellent evidence from Europe and Canada that these sites save lives, clean up the streets and parks, and offer people with addiction information on how to access treatment. The idea has support from our local paper, too. Now, will it actually happen here? Currently there are zero in the USA. We are, as a society, very paranoid about “enabling” risky behavior by making it safer.
- Wonder why oral vancomycin is still so expensive despite having multiple generic manufacturers? Me too — the linked review covers this unique (and frustrating) situation, one that highlights the oddity of drug pricing in the USA and hints at price collusion.
- Worthy long-read: This fascinating profile of AIDS Healthcare Foundation’s director, Michael Weinstein. Ironically, the piece reminded me of another powerful person in the annals of HIV treatment, only in a completely different area — organic chemist Ray Schinazi. Both elicit strong reactions, pro and con.
- The 2019 Conference on Retroviruses and Opportunistic Infections (CROI) will take place in Seattle, March 4-7. The date and location of this important meeting used to be the best-kept secret in Infectious Diseases, which made creating academic schedules a nightmare. Vastly prefer this advance notice!
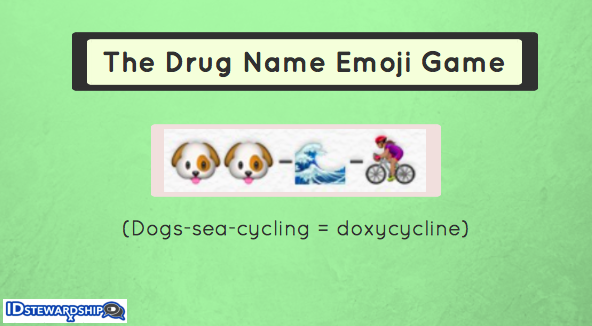
- This drug name emoji game is awesome. I selected every ID doctor’s favorite antibiotic for display up at the top of the post. It should prove especially useful for this bad upcoming tick season. And imagine if this is how we had to write prescriptions.
OK, now for a quick non-ID section:
- Here is a spot-on criticism of the “Milestones” process for evaluating medical residents and fellows. These ACGME Milestones offer a perfect example of how quantifying skills for which there are no reliable metrics leads to distracting noise, not real data. We got much more valuable information in the past when resident evaluations were done by narrative, using actual language rather than checking boxes.
- The New York City Department of Public Health keeps meticulous records of dog names. Bella is right now #1 for females, Max for males. And it looks like Nellie is very popular in my parent’s neighborhood. Just so you know.
https://twitter.com/HelenBranswell/status/860553983718563845
- If you have even the slightest interest in baseball, I strongly recommend the book Smart Baseball, by Keith Law. Did you know that batting average, RBIs, and pitcher wins are highly flawed measurements of a player’s ability? And that you should never bunt solely to advance the runner? That the pitcher “save” is an evil force in the game? All 100% true, and clearly and entertainingly explained. I can also strongly endorse the author’s excellent blog, where he writes about books, food, movies, and is a consistent and forceful defender of the importance of vaccines for personal and public health. We like that!
- The television series “American Crime” is outstanding, but hardly anyone is watching. Despite critical acclaim, the ratings are only fair — seems like a quality/ratings ratio on par with “Friday Night Lights,” another terrific series that was relatively ignored when it aired. The linked piece places some of the blame on the rather blah and misleading title, a very good point.
Hey, I started with CRISPR/Cas9, so here’s the big finish. Take it away, A Capella Science!