An ongoing dialogue on HIV/AIDS, infectious diseases,
October 15th, 2017
The Best Antiretroviral Therapy for Pregnant Women? The Controversy Continues
There’s considerable controversy in an area of HIV medicine that one would think should be all but solved by now.
It’s what HIV treatment we should give pregnant women.
The issue isn’t how to prevent the virus from being transmitted to the newborn — suppress the virus in mom, baby doesn’t get it — it’s what’s safest for the pregnancy outcome.
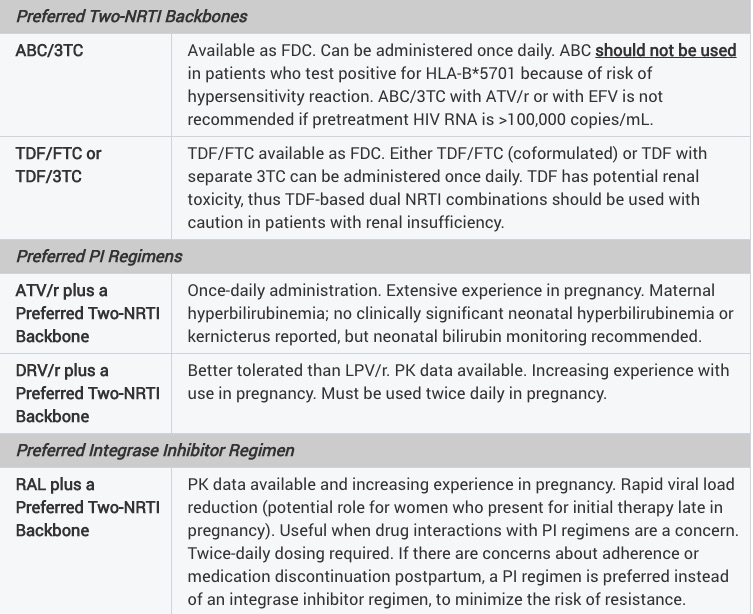
The uncertainty spills over to the HIV perinatal guidelines, which are notable for how different they are from those for non-pregnant adults:
Taken literally, these guidelines would support a regimen of ABC/3TC and twice-daily DRV/r for a pregnant woman — something we’d never prescribe a non pregnant treatment-naive patient for several obvious reasons.
Additionally, there is only one integrase inhibitor-based regimen — TDF/FTC, raltegravir — while INSTI-based regimens dominate the general recommendations for HIV treatment. There just aren’t enough data yet on the use dolutegravir in pregnancy, though we did see some encouraging information during the IAS meeting this summer. And information on tenofovir alafenamide are scant.
Now, potentially making HIV treatment during pregnancy even more divergent from standard-of-care, non-pregnancy treatment, comes a surprising new set of recommendations from the British Medical Journal.
Entitled Antiretroviral therapy in pregnant women living with HIV: a clinical practice guideline, the paper recommends using zidovudine/lamivudine over tenofovir DF/emtricitabine during pregnancy:
Here’s their stated reason for this surprising recommendation:
Tenofovir and emtricitabine probably increase the risk of early neonatal death and preterm delivery <34 weeks compared with zidovudine and lamivudine; this is more certain when they are combined with lopinavir/ritonavir.
Importantly, most of the data they cite in support of this recommendation come from the PROMISE study, which did show higher rates of very preterm delivery before 34 weeks and early infant death with TDF/FTC compared with ZDV (a.k.a. AZT)/3TC. But an important caveat is that the drugs were given with LPV/r at high dose, a regimen we rarely use today, and which substantially increases tenofovir levels.
The authors of the PROMISE study themselves responded to the BMJ piece, stating that they do not agree with the recommendation to use ZDV/3TC over TDF/FTC for several reasons. While I encourage people interested in this topic to read their full comment, they cite important observational data on the use of TDF/FTC/EFV:
Compared with a regimen of TDF-emtricitabine (FTC)-EFV, all other regimens, including AZT-based ART, were associated with higher risk of adverse outcome; increased risk of preterm birth, very preterm birth and neonatal death were observed for infants exposed to AZT-lamivudine (3TC)-lopinavir-ritonavir.
Plus, we can add to these reassuring data a new paper, just published in the Journal of Infectious Diseases. In a prospective evaluation of 422 pregnancies, the researchers found that TDF was not associated with adverse perinatal outcomes, and that preterm birth occurred less frequently among pregnancies exposed to TDF.
My take? We know from thirty years (yep, it’s been that long) of experience that ZDV has considerable toxicity — subjective side effects such as nausea and headache, and additional problems related to mitochondrial toxicity, including bone marrow suppression, lipoatrophy, and lactic acidosis.
As a result, it’s very hard to imagine prescribing zidovudine again under any circumstances, including during pregnancy. Today, the most commonly used initial regimen at our hospital during pregnancy is TDF/FTC and raltegravir; if patients are on a successful treatment and become pregnant, we generally continue that, almost regardless of what it is.
And we eagerly await the results of a three-arm study in pregnant women, led by my colleague Shahin Lockman, which is just getting started. It compares TAF/FTC plus dolutegravir, TDF/FTC plus dolutegravir, and TDF/FTC/EFV.
This, we hope, will move treatment of pregnant women with HIV closer to the treatment we offer non-pregnant adults.




I think that the PROMISE results apply maybe to Africa but not to most of the rest of the world. I would leave an HIV-infected woman who becomes pregnant on the regimen that has her suppressed and use a DTG-based regimen + TDF/FTC in one starting therapy but AZT….never!
Damnit, Jim, I’m a lawyer not a doctor, but if the only reason TAF is not part of the guideline is the lack of data that will soon be forthcoming, and the concern over tenofivir levels came from a study that used a TDF + booster, wouldn’t common sense dictate TAF + 3TC/FTC+ RAL or DTG? Lower tenofivir levels than even an unboosted TDF regimen, and INSTI that does not (from my read of this article) come with data suggesting additional perinatal morbidity? And who would want to give a booster to a pregnant woman anyway, with all the potential interactions with other medications that may become nececessary as part of prenatal care or during delivery? Isn’t this exactly the type of scenario where common sense + informed consent come into play?
Hi Paul–as you know, this is my research focus. The reality is that good data for drug safety in pregnancy is lacking for many of our commonly used ARVs and many of our recommendations for ART in pregnancy are based on clinical experience. Despite the need for more data in this area, I was appalled that this article got past reviewers and was published. There are so many flaws, it’s hard to know where to start. It’s clearly not a meta-analysis, but just takes the results from one study, PROMISE, and generalizes. The PROMISE authors have written a really good reply, but for those who don’t think think all the time about studying ART safety in pregnancy, a few more points.
1. For starters, ‘is TDF/FTC or ZDV/3TC safer in pregnancy’ is the wrong question. The primary unit of analysis of analysis for the study of ART safety in pregnancy should be ART regimen. ART is (almost always) three drugs in combination. ARVs can interact with each other within a combination. PROMISE showed us that when combined with LPV-r, TDF/FTC may not as safe as ZDV/3TC (at least when LPV-r is doubled in the third trimester with TDF/FTC, as it was in PROMISE)…but we also have a good amount of evidence that when combined with EFV and NVP, TDF/FTC is likely safer in pregnancy. We may eventually find that certain combinations or individual ARVs are unsafe in pregnancy, but this can only be seen using data that includes multiple different ARV combinations (in large enough sample sizes to draw conclusions).
2.”ZDV/3TC vs. TDF/FTC’ is also the wrong question because we shouldn’t be using ZDV/3TC for first-line ART anymore (for medication toxicity and tolerability issues rthat you’ve pointed out). The public health implication would be that we should not use any fixed dose combinations, or first-line ART in women of childbearing age (since we need to change regimen prior to pregnancy to ensure that there is no toxic effect). Sometimes, we get so caught up in the potential or theoretical direct fetal effects of a medication, that we forget that there can be serious adverse fetal outcomes if the mother is under-treated (ie more effective drugs inappropriately withheld during pregnancy)– for example becoming viremic (MTCT), anemic (preterm delivery), having lactic acidosis (miscarriage, stillbirth) during pregnancy as might be expected if ZDV/3TC were used widely. Whether there is any difference in safety of TDF/FTC vs. ABC/3TC vs. TAF/FTC during pregnancy is the only clinically relevant question currently.
3. Pregnant women are not included in initial drug RCTs, so the vast majority of information on the safety of medication in pregnancy comes from observational studies (in HIV we are lucky to have a few RCTs as well). This is the reality, and therefore it’s hugely important to take into account the observational data (to evaluate for strength of methodology, different types of bias, applicability to other settings). Ignoring observational data when making broad pregnancy treatment recommendations, as was basically done in this study, is ignoring the bulk of evidence on the subject and is outrageous–and again easily leads to treatment recommendations that could hurt mothers and their babies.
I think these issues of asking the right question, and using all available evidence (not just RCT) are more obvious if you imagine that instead of drawing the conclusion that ZDV/3TC was safer than TDF/FTC in pregnancy, this paper had drawn the conclusion that ZDV/3TC/LPV-r was safer than TDF/FTC/LPV-r (which is what the analysis actually evaluated) and then offered a practice guideline that said ZDV/3TC/LPV-r should be the first-line ART regimen for all HIV-infected pregnant women. I am fairly certain that it would not have been published, and would be rejected by almost all HIV providers around the world (even if the best RCT data we have right now for is for ZDV/3TC/LPV-r).
It’s relatively easy these days to do a meta-analysis in the place of a well-designed study, and I wonder if the authors of this publication were not involved enough in research or clinical management of HIV-infected pregnant women to realize that their research question and study design could actually have a negative impact on maternal and child health.
I am afraid I and my fellow HIV physicians are stumped by the obvious reasons not to use Darunavir/ritonavir with abacavir/3TC for pregnant women. Besides the allergy and going to twice daily dosing for darunavir, what are some of the other reasons.
Thank you
D.R.
Hi DR, thanks for your question.
The best regimens for non-pregnant treatment-naive patients are all very simple (1-2 pills), with very rare side effects. We would never choose to start a non-pregnant patient on abc/3tc, and twice-daily darunavir/ritonavir — more complexity, more side effects (esp. the GI side effects of 200 mg/day of ritonavir), more metabolic abnormalities, more drug interactions. In addition, there are hardly any data on that regimen from treatment-naive clinical trials.
Paul
Please note that the US Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission fully considered the available evidence, including the PROMISE trial results, and concluded that the assessment of expected benefits and harms favored TDF/FTC over ZDV/3TC, leading the Panel to keep TDF/FTC as a Preferred recommendation and ZDV/3TC as an Alternative recommendation for antiretroviral-naive pregnant women living with HIV in the United States. Please see:.https://aidsinfo.nih.gov/guidelines/html/3/perinatal/0
These US Guidelines also support Dr. Sax’s general advice that: “In most cases, women who present for obstetric care on fully suppressive ARV regimens should continue their current regimens.” Please see: https://aidsinfo.nih.gov/guidelines/html/3/perinatal/488/overview.
A full update of these guidelines is anticipated in the coming weeks.
When you say, “We know from thirty years (yep, it’s been that long) of experience that ZDV has considerable toxicity — subjective side effects such as nausea and headache, and additional problems related to mitochondrial toxicity, including bone marrow suppression, lipoatrophy, and lactic acidosis,” aren’t you now agreeing with what Peter Duesberg has been saying since 1987? And validating what Nobel Laureate Kary Mullis wrote in 1996 when he wrote, “We [he and Duesberg] have also not been able to discover why doctors prescribe a toxic drug called AZT (Zidovudine) to people who have no other complaint than the presence of antibodies to HIV in their blood. In fact, we cannot understand why humans would take that drug for any reason,” in the foreward to Inventing the AIDS Virus?
The original 1.5 gram per day dose promptly caused neutropenia and anemia (this was noted even in the one, flawed, brief study upon which its hurried approval was based). Concorde later found patients treated with 1.5 grams per day had a 25% increased relative risk of death than 600 MG per day. So, if the drug suppresses bone marrow and causes neutropenia, then doesn’t the drug cause an acquired immune deficiency syndrome, which Duesberg called “AIDS by prescription?”
Can we at least now all agree that Duesberg was correct to argue against Zidovidine monotherapy in addition to correctly predicting the HIV couldn’t work through a directly cytocidal mechanism because it doesn’t infect enough T-cells? I don’t think Duesberg has everything right either, but it’s important to clear the air on Zidovidine given WHO still lists it as an Essential Medicine and the epidemiology of AIDS was skewed by AZT causing the very condition it was prescribed to treat.