An ongoing dialogue on HIV/AIDS, infectious diseases,
July 24th, 2016
Really Rapid Review — AIDS 2016, Durban
 The International AIDS Conference returned this year to Durban, South Africa, where it was famously first held in 2000. At that time the HIV epidemic was exploding in South Africa; funding for HIV treatment was essentially non-existent, and there was ongoing HIV denialism quite openly from some very influential figures in the South African government (including the President). Globally, fewer than 1 million people were receiving antiretroviral therapy, hardly any of them in Africa.
The International AIDS Conference returned this year to Durban, South Africa, where it was famously first held in 2000. At that time the HIV epidemic was exploding in South Africa; funding for HIV treatment was essentially non-existent, and there was ongoing HIV denialism quite openly from some very influential figures in the South African government (including the President). Globally, fewer than 1 million people were receiving antiretroviral therapy, hardly any of them in Africa.
Encouragingly, according to this UNAIDS report, the number being treated today is 17 million — with, incidentally, the largest number in South Africa. Yes, this 17 million is only half the number who need treatment, but this is still extraordinary progress. HIV-related deaths started steadily declining in 2005, a trend one can hope will continue.
OK, on to a Really Rapid Review™ of the conference. It’s organized by prevention, treatment, complications, and whatever else happened to have caught my eye; I welcome suggestions for what I’ve missed (undoubtedly something important) in the comments section.
- In the open-label extension of the IPERGAY study, the efficacy of the “on demand” PrEP was 97%. There was only 1 seroconversion out of around 300 participants, this in a patient with no detectable blood levels of tenofovir. The average number of pills taken/month was 18, so it doesn’t quite answer the question of whether this strategy works for people whose “on demand” is quite a bit less frequent than in the study participants (say, once monthly or even less often).
- In 1013 serodiscordant couples in Kenya and Uganda, 6 months of PrEP to seronegatives and ART to the those with HIV reduced HIV incidence 95% compared with historical controls. All 4 incident HIV infections occurred in people not taking PrEP, or with a source not on ART (outside the couple). Interesting question — should we really be recommending PrEP indefinitely to HIV negative people in monogomous serodiscordant relationships if their partner has virologic suppression? Our guidelines say so, but I’m dubious.
- Nearly 80,000 people have been prescribed PrEP in the USA, with over a 700% increase since 2012. The really sharp upward inflection started after presentation of the PROUD and IPERGAY studies in 2014, and not surprisingly occurred mostly in men. Fascinating geographic distribution: New York state with the largest number of scripts, then California; Massachusetts with the highest rate of prescribing based on population. Southeast USA — where HIV incidence is highest — unfortunately lags way behind, clearly a practice gap worth improving.
- A study of PrEP in at-risk 15-17 year old male adolescents showed just how hard this population will be to reach. The study pre-screened nearly 3000 peole to find 260 eligible. 152 refused participation. 108 were screened. Finally, 79 enrolled — and then 32/79 (40%) stopped the study before 48 weeks! Adherence also sharply declined, and HIV incidence was 6.4/100 person/years (that’s very high). Clearly some other strategy needed.
- The risk for drug resistance with PrEP in 5 clinical trials was only 0.05%. Even when inadvertently prescribed during acute HIV, the risk is “only” 37% — lower than I would have expected. It’s mostly M184V, of course — a mutation that would be easy to salvage.
- Could the vaginal microbiome partially explain the lower efficacy of PrEP in women? (Link is to several presentations.) Seems that Gardnerella and Prevotella spp may inactivate tenofovir more than lactobacilli. If this isn’t an ID nerd’s factoid, I don’t know what is.
- A vaccine strategy demonstrated impressive “correlates” of HIV protection. Results will allow a large efficacy study (HVTN 702) to go forward in South Africa. For the record, the strategy is called “clade C ALVAC-® (vCP2438) and Bivalent Subtype C gp120/MF59®”. These vaccine researchers sure do have their own special language.
- The PARTNERS study of “condomless sex” (published last week in JAMA) will follow MSM participants through 2018. The rationale is to provide a more precise estimate of the risk of acquiring HIV in this highest risk group, a very important remaining question.
- In a randomized study of immediate (same day) vs standard of care timing of ART in Haiti, early therapy improved survival. Important definitions: “immediate” = same day as HIV diagnosis; standard-of-care = only 21 days later, with counseling visits before then. This is a remarkable result, especially since 1) patients with active OIs were excluded; 2) the number of clinic visits was the same; and 3) the effect was so great a DSMB stopped the study early. Seems that starting ART right away improves engagement in care, and all of those “are they ready to start ART?” questions have been answered: YES THEY ARE.
- In the ARIA study, ABC/3TC/DTG was superior to TDF/FTC + ATV/r in treatment naive women. There were both fewer discontinuations for adverse events and fewer virologic failures in the ABC/3TC/DTG arm. The integrase-first strategy wins again, with a very similar outcome to the WAVES study, which used ECF-TAF and was blinded.
- In LATTE-2, an every 4 week schedule of cabotegravir and rilpivirine injections had fewer virologic failures than every 8 weeks at 48 weeks. One of the participants in the q8 week arm developed resistance to both rilpivirine and cabotegravir. Note that while both strategies were comparable to oral therapy, the q4 week approach will be used in the phase 3 studies based on these results. And in a funny mix-up showing how small the HIV research world is, the presenting author David Margolis was introduced as the other David Margolis. Bet the two John Bartletts have had a similar experience.
- In newly diagnosed patients with advanced HIV disease (CD4 < 100), adding raltegravir to standard ART did not improve clinical outcomes. Once again, 4 drug ART was not better than 3, though as expected HIV RNA declined faster and CD4 increased more in those receiving RAL. Called the REALITY study, this was an extraordinarily ambitious (sorry for the cliche, but it’s true) trial, conducted in multiple African countries and with 3 randomizations with a factorial design: intensive ART (this part), enhanced OI prophylaxis (see next bullet), and nutritional intervention (not presented here). This is the largest study done in advanced HIV disease, a group for whom we still have lots of questions since early mortality is so high.
- In this same vulnerable population with advanc
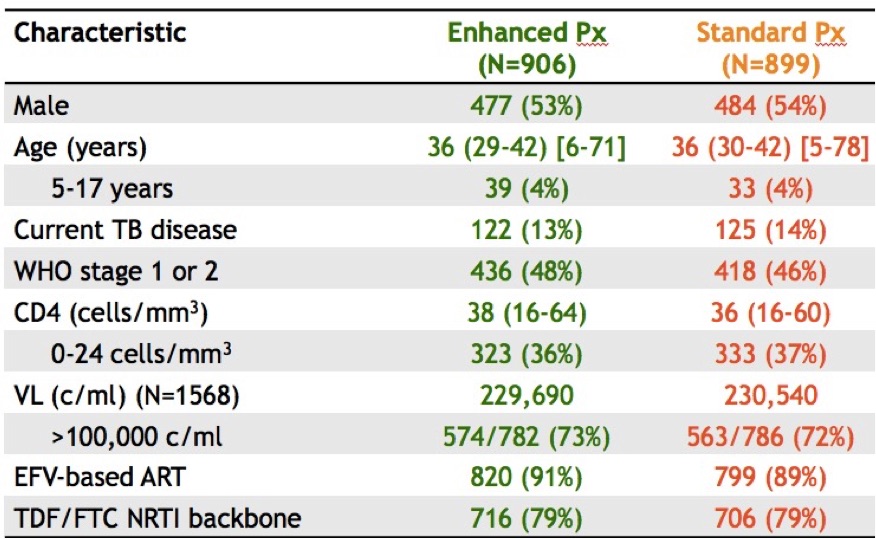 ed disease, enhanced OI prophylaxis improved survival. In addition to ART, the control arm received TMP/SMX (+/- INH depending on that local policy); the enhanced group received ART plus TMP/SMX, INH, fluconazole for 12 weeks, azithromycin for 5 days, and a dose of albendazole (why not). And take a look at those entry criteria! Mortality at 24 weeks: 8.9% vs 12.2% (p< 0.04), benefits sustained at week 48. These results could change treatment guidelines for this region; the number needed to treat/live saved was only 30. For the record, my friend/colleague Trip Gulick had a similar idea for a study of “pan-prophylaxis” in the USA — in 1991 (5 years before effective ART).
ed disease, enhanced OI prophylaxis improved survival. In addition to ART, the control arm received TMP/SMX (+/- INH depending on that local policy); the enhanced group received ART plus TMP/SMX, INH, fluconazole for 12 weeks, azithromycin for 5 days, and a dose of albendazole (why not). And take a look at those entry criteria! Mortality at 24 weeks: 8.9% vs 12.2% (p< 0.04), benefits sustained at week 48. These results could change treatment guidelines for this region; the number needed to treat/live saved was only 30. For the record, my friend/colleague Trip Gulick had a similar idea for a study of “pan-prophylaxis” in the USA — in 1991 (5 years before effective ART). - Updated 48-week results of the dolutegravir plus lamivudine (PADDLE) study had 1/20 with virologic failure. This is a single-arm pilot trial for patients with screening HIV RNA < 100k. Notably, the one with virologic failure had entry HIV RNA > 100K and was suppressed initially, but developed low-level viremia from week 36-48. No resistance was detected, and he re-suppressed on DTG-3TC and now is on triple therapy. (A second patient died from suicide before week 48 for an overall efficacy of 90%.) We need larger studies of this strategy in a broader patient population and with longer follow-up before it is widely adapted.
- A novel formulation of once-daily raltegravir was non-inferior to standard twice daily dosing. In this randomized, double-blind trial, the once daily arm was dosed as two 600 mg tablets daily, and all subjects received TDF/FTC. While overall outcomes were virtually identical (and excellent) in both arms, the once-daily arm had 5 cases (0.9%) of emergent resistance vs zero in twice daily arm. Interestingly, both arms had a lower rate of resistance than in prior randomized studies of raltegravir in naive patients.
- In START, the patients who benefited the most from early ART had HIV RNA > 50,000, or CD4:CD8 ratio < 0.5, or age > 50, or Framingham risk scores > 10%. These individual characteristics brought the number needed to treat for benefit of early ART (CD4 > 500) down from 128 to 40-50. Important fact for stat geeks — this was a univariate analysis.
- In the PROMISE Study, pregnant women with high nadir CD4s who continued ART post delivery had better clinical outcomes than those who stopped therapy. This was an important clinical question when this study was designed in the late 2000s, but the comparison had to be stopped early when the START study results became available last year. While the primary endpoint (AIDS, severe non-AIDS events, death) showed no difference between arms, those who continued had fewer other manifestations of HIV disease — TB, bacterial infections, zoster, mucosal candidiasis.
- Efavirenz again associated with suicidality in patients starting ART. This complex analysis (also from the START study) had to account for the fact that investigators avoided EFV-based regimens in participants with psychiatric disease (“channeling”), so in fact those receiving EFV had a lower rate of suicidality than those who did not. However, the rate was higher comparing EFV treated subjects to those in the deferred arm; this was not observed in other regimens. Given the results of the published ACTG study with similar findings, I would certainly avoid initial EFV-based therapy in those with a history of depression (and probably pretty much everyone if you have other options).
- In the ASTRAL-5 study, 12 weeks of velpatasvir/sofosbuvir for HIV/HCV co-infection cured 95% of study participants. This is a pan-genotypic regimen, a terrific new option for genotypes 2 and 3 in particular. Note that it cannot be given with efavirenz, and that if your patient is on TDF, a switch to TAF makes sense just as it does for ledipasvir.
- In the TURQUOISE-I, Part 2 Study, “PROD” (+/- RBV) cured 97% or more of those with HIV/HCV coinfection. (PROD = parataprevir, ritonavir, ombitasvir, dasabuvir.) Despite the relatively high pill burden and need for RBV in genotype 1a, this is a very effective regimen. Cumbersome study name, though. Couldn’t it have been “Turquoise 2”? Reminds me of this funny post on movie titles, probably could to the same thing for study names.
- Adjunctive therapy with vorinostat, maraviroc, and hydoxychloroquine did not decrease time to virologic rebound or reduce the size of the latent reservoir. This small randomized trial was conducted in patients treated during acute HIV infection — hence those most likely to benefit from cure interventions. A well done study, but probably one of many “negative” studies done in this area we’ll see over the next few years.
A few non-scientific words about being back in Durban after 16 years:
- Underrated beachfront. There’s plenty of activity during the day, with the surfers out between 6-7AM and the walkers, joggers, bikers, skateboarders, rollerbladers, and general observers appearing just a bit later to experience the beautiful sunrise. All day long a walk on the beachfront promenade was an ideal way to clear the brain of “conference head.”
- Great, affordable food. Not surprisingly, there is a pervasive Indian influence, as Durban has one of the largest Indian populations in the world outside of India. (If you get a bunny chow, be reassured it has nothing to do with rabbits.) And don’t skip the Pinotage and Chenin Blanc (I didn’t). Unfortunately, unlike my experience 2 years ago in Melbourne, the coffee is terrible.
- Pride. Every person I met from Durban was both extremely kind and extraordinarily proud of their city; all knew the history of the city well, and were eager to talk about it. I sensed a bit of both envy and disapproval of both Cape Town and Johannesburg.
- Uber rules. Cheap, reliable, and every bit (if not more) the “disruptive innovation” it is in the USA.
- Safety. It did seem as if the local advice about security was even stronger than the first time I visited. It was obvious stuff: don’t walk alone to the conference center, don’t carry your computer, don’t take out your cell phone on the street, and (repeatedly) never walk alone at night. Some of this, no doubt, is that the first time we were here it was pre-9/11. It’s a different world.
An ancillary benefit about going to this conference — the noise from a certain political convention was only a faint peep, or a footnote on a distant TV that happened to be turned to CNN!


Drugs, drugs, more drugs – for treatment and prevention – great.
What about overcoming impediments to diagnosis and treatment?
What about overcoming stigma, ignorance, misinformation and changing attitudes and behaviors?
Any successful programs applying interventions based on local psychology, sociology, and/or anthropology?
Thanks.
Lots of exciting PrEP news indeed.Flying from the U.S. to Durban (and back) is not for the meek and mild-mannered however. Wow that was a long flight.
“If this isn’t an ID nerd’s factoid, I don’t know what is.” How about it also being an ID nerd’s groupie’s factoid? I will have to figure out how to drop that little fun fact into a lecture.
Thank you for the really rapid review! It was a pleasure to be there (even with a tremendous jet-lag).
I would only add, that we had a wonderful pre-meeting symposium on Cure, co-chaired by Françoise Barré-Sinousi of course along with Steve Deeks and Sharon Lewin.
Afterwards, during the meeting there were several sessions focused on the reservoir elimination or cure strategies. I would only call your attention on the project EPISTEM. The European project to investigate the potential cure of HIV by Stem Cell Transplantation (http://www.epistem-project.org). It was presented on the session “Targeting Reservoirs for Cure”. They report 3 patients with Stem-cell transplant (all 3 had a clinical condition that required transplant), one of them has no reservoir detected! The authors highlight the importance of a graft vs host disease (or as they called it the “Graft Vs Reservoir”) in order to eradicate the reservoir. Of course none of these patients have stopped therapy (yet).
So let’s wait and see…