An ongoing dialogue on HIV/AIDS, infectious diseases,
December 20th, 2014
New HCV Option Effective, Safe, Well-Tolerated — And Use Will Likely Be Driven by Payors
As expected, the FDA approved the next treatment option for HCV on Friday — “Viekira Pak”, a (sometimes complete) regimen consisting of ritonavir-booted parataprevir and ombitasvir given as a two pills once a day, plus one pill of of dasabuvir given twice daily. It is indicated for treatment of HCV genotype 1.
For those of you mechanistically inclined, parataprevir is a protease inhibitor, ombitasvir an NS5A inhibitor, and dasabuvir the first approved non-nucleoside polymerase inhibitor. Ritonavir is there for PK boosting.
Cure rates have been outstanding — 90% and higher — and severe adverse events rare. All good news so far.
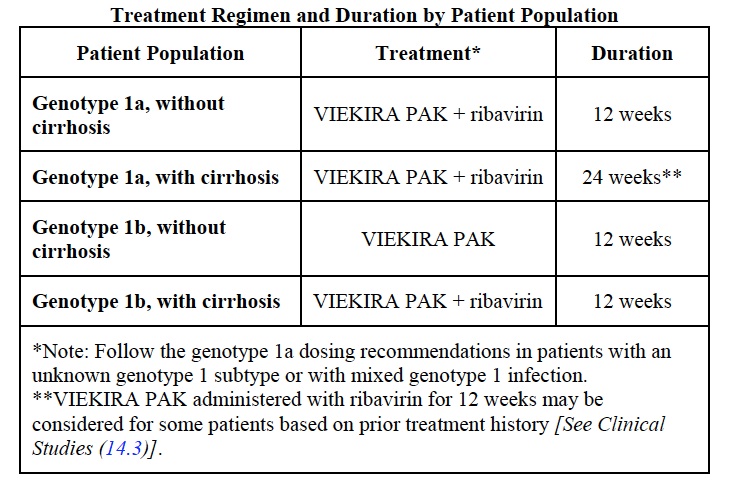
So what’s the issue? The full prescribing information is here, but this is the key table:
Obviously, many patients will require concomitant administration of ribavirin, which makes this a considerably more complex regimen than the sofosbuvir/ledipasvir combination pill that has been available since October. Two other disadvantages include more drug-drug interactions (ID/HIV doctors are certainly familiar with ritonavir), and the potential for broader antiviral resistance if the treatment fails.
Whether these make a difference or not in clinical practice is a key unknown. But you can bet good Hanukkah gelt that payors are highly motivated to find out. Here’s some proof.
Hey, Happy 20th Birthday to this classic:


All this will do is create more work for my staff and me with another round of prior authorizations that will be denied.
I think the observations under estimate the many disadvantages of Ziekira + ribavirin versus Harvoni in terms of inconvenience, toxicity ( ribavirin), liver monitoring and longer duration of treatment for many patients.
Keith, that was my point about emphasizing the ribavirin. It greatly adds to the complexity of the regimen, and will be required for most patients.
Paul
Paul:
I understand.
Thanks!