November 16th, 2016
Before You Do Anything Else, Take a Deep Breath
Elizabeth Donahue, RN, MSN, NP-C
Exactly one week ago I arrived at my office in a stupor that it seemed even my favorite coffee shop could not fix. I had not slept more than two hours the night before, and on top of being tired, I was emotional. I had spent too much time in the wee hours of the morning reading post-mortems of the campaigns, a myriad of predictions and possible next steps, and receiving messages from friends who felt that they suddenly had something to lose or to fear. I felt a bit like something major had happened and yet here we all were reporting for our daily work like nothing had or would change. I literally thought to myself — how will I get through this day?
When you spend your days helping others address their physical and mental health concerns, it provides you with the kind of perspective that few are lucky enough to have day to day. It affords you the privilege of knowing the very personal stories of hundreds of people who trust and confide in you. It regularly serves as a reminder of the tenacity of the human spirit, obstacles that can be overcome, and struggles that are endured. Not every story has a happy ending of course, but they all provide perspective and I consider that a gift.
Let me pause here and say that I love my country and I respect our democracy. I also know that some people experienced the results differently than those in my inner circle and I have taken care and consideration to understand their feelings. Furthermore, I have gained some perspective in the last week. While the dust is settling, factual new information has come through, politicians are seeming to come together to move forward, and the stock market is rebounding from a dramatic plunge — and these things have somewhat lessened what seemed like a great loss. But last week, loss is exactly what I felt and I wasn’t sure how I was going to deal with that. Then my patients started arriving, and they felt it too, and it became instantly clear that my new role was to worry less about myself and figure out how to take care of them.
My first patient had a fairly benign concern, but she was also recently diagnosed with MS. She now has a pre-existing condition and is worried about what that means for her future ability to receive affordable health care. My next patient presented for a routine physical. When my medical assistant asked her if she had any specific concerns or questions about the day, she became emotional and said she wanted to talk about her anxiety related to the election. She is a recent graduate, has a new government job, and is now worried about keeping that job and how she will be treated in a male-dominant office. In another routine physical after that, a young woman requested to have an IUD placed because she feels uncertain about her ability to obtain and afford reliable birth control going forward. The woman after her earnestly asked me if she would lose her health care coverage because she is under 26 and currently on her parent’s healthcare plan thanks to the ACA, which has been targeted for repeal. My morning went on and on like this — concern after concern about very real ways in which patients have already been and may continue to be affected by an unprecedented campaign and transition. During lunchtime, a woman who cleans our office, and with whom I’ve become friendly, talked about how she is worried about her friends and family members (from her native Honduras) facing discrimination and hatred.
To all these people, I wanted to shout, I’M WORRIED TOO. But I had to realistically consider my next steps; what can I tell them? Moreover; what can I tell them when I don’t have any of the answers to their questions? Their concerns related to their health care are real and deserve a response. In medicine, we are used to doing the opposite — diagnosing an issue, making a plan, communicating next steps to a patient. We don’t have all the answers all the time, of course, but we usually at least have a strong differential diagnosis and a plan to figure things out.
So during a time when I have also been disappointed, anxious, uncertain, angry, and full of questions (you name it, I’ve felt it in the last week), I’ve developed sort of a new mantra: Take a deep breath. I might say this any number of times during a regular day in clinical care (when a cough needs to be evaluated, before an injection, when stitches are coming out, etc). But now I’m saying it with a different meaning. So, much like when something seems wrong but the diagnosis is not clear, I’m saying to my patients, “I hear your worry. Your concerns are real. We don’t have the answers right now. So don’t forget to breathe — take a deep breath — it may take time, but we will figure it out.”
I think everyone needs to do this in the wake of a very divisive election. This is not advice that is exclusive to my young, female patients who feel afraid because of their age, gender, race, employment status, etc. When tensions and emotions are running high — whether in a medical emergency or in the current political climate — before we act impulsively, before we say something that might be hurtful to another, before we jump to conclusions and judge, let’s all take a collective deep breath. After that breath, the next steps may be clearer. You might decide to refer your patients for the care that they are worried they may lose (I have done this a few times in the last couple of days); you might decide to volunteer for a cause you believe in; you might decide to engage in conversation to help you better understand what you are feeling or what someone else is feeling. I think it will help us all to start to understand and heal. Only then can we move forward in a productive and thoughtful way.
November 14th, 2016
Fall Changes in the U.K.
Megan Tetlow, PA-C

Megan Tetlow, PA-C, is from Fort Myers, Florida, now working in Sheffield, England, as part of the National Physician Associate Expansion Program. She practices in gynecologic oncology and is a guest blogger for In Practice.
Autumn is in full swing here in the U.K., and having lived in beautiful but season-less south Florida for the past several years, I am mildly obsessed. Even in the city, the fall colors are breathtaking. Personally, I have been really enjoying boots, hot tea, and weekend hikes in the countryside (and in the U.K. fashion, always ending in a cozy country pub). With all of these seasonal changes around, I’ve been thinking about some of the changes I’ve made to how I practice since arriving to the U.K., and about the differences between practicing as a PA in the U.K. and in the U.S.
Leave Your White Coat at Home
You won’t see any white coats walking around the floors of U.K. hospitals. This is not a PA vs. physician division: no healthcare providers in the U.K. wear white coats, docs included. I asked around about what patients might think if you walked into an exam room while  wearing a white coat. The answers usually fell along the lines of patients wondering why a scientist was seeing them at their doctor’s office, or why they were being seen by an actor who plays a doctor on an American television show. Not only are white coats not worn as a general practice, they are actually not allowed. More specifically, you are not allowed to wear anything long-sleeved. The National Health Service has a policy of “bare below the elbows” when providing patient care. The purpose of this is to decrease the rate of infection and contamination between patients. Thinking back about all of the things my white coat saw between washings, it’s probably a really good idea. Though I do sometimes wistfully long for all those pockets…
wearing a white coat. The answers usually fell along the lines of patients wondering why a scientist was seeing them at their doctor’s office, or why they were being seen by an actor who plays a doctor on an American television show. Not only are white coats not worn as a general practice, they are actually not allowed. More specifically, you are not allowed to wear anything long-sleeved. The National Health Service has a policy of “bare below the elbows” when providing patient care. The purpose of this is to decrease the rate of infection and contamination between patients. Thinking back about all of the things my white coat saw between washings, it’s probably a really good idea. Though I do sometimes wistfully long for all those pockets…
Assistant vs. Associate
As I’ve mentioned in previous posts, PAs here in the U.K. are called physician associates as opposed to physician assistants. Interestingly, they were previously called physician assistants here as well, but the U.K. Department of Health actually recommended they change the name to avoid confusion with other professions — for example, personal assistants, which are administrative personnel in the U.K. This is a sentiment I’m sure many of my colleagues, British and American, could appreciate. Just like the old song, “You like to-may-to and I like to-mah-to,” I say assistant and they say associate. Personally, I rather like the term physician associate, but that’s a larger conversation for perhaps another day.
Prescribing in the U.K.
Finally, currently in the U.K., physician associates cannot prescribe medication (including ordering medications for inpatients as well as for outpatients) or order ionizing radiation (which includes x-rays and CT scans). I had debated whether to include this, but I ultimately did because (a) it highlights a big difference in how PAs currently practice in the U.K. versus the U.S. and (b) I have received several questions about i t. In order for PAs in the U.K. to have that authority, physician associate must first become a regulated profession and then be added to the list of healthcare providers that are allowed to prescribe. Both of these actions require parliamentary acts. It’s a top priority of the Faculty of Physician Associates (the U.K. counterpart to the American Academy of PAs) to become a regulated profession, and this is something they have been working very hard to achieve. So while yes, right now, PAs cannot write prescriptions or order CT scans, it is my belief that it is not a question of whether PAs will ever be allowed to do these tasks, but rather when. I have never heard anything but complete support from doctors and other healthcare providers here that PAs should be allowed to do both.
t. In order for PAs in the U.K. to have that authority, physician associate must first become a regulated profession and then be added to the list of healthcare providers that are allowed to prescribe. Both of these actions require parliamentary acts. It’s a top priority of the Faculty of Physician Associates (the U.K. counterpart to the American Academy of PAs) to become a regulated profession, and this is something they have been working very hard to achieve. So while yes, right now, PAs cannot write prescriptions or order CT scans, it is my belief that it is not a question of whether PAs will ever be allowed to do these tasks, but rather when. I have never heard anything but complete support from doctors and other healthcare providers here that PAs should be allowed to do both.
So, that’s an intro course on delineating the differences between PAs on either side of the Atlantic. Now if you’ll excuse me, there are some country pubs and fall foliage to be seen. Also, it is becoming starkly apparent that this Florida girl does not actually own a winter coat.
November 2nd, 2016
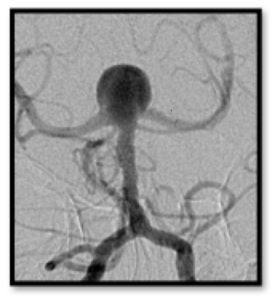
Intracranial Aneurysms for the Non-Neurosurgical Provider: Primer Series (Part 2)
Bianca Belcher, MPH, PA-C
In Part 2 of this primer series on intracranial aneurysms for non-neurosurgical providers, I will discuss modifiable and non-modifiable patient risk factors for subarachnoid hemorrhage (SAH) during intracranial aneurysm treatment.
Modifiable Risks:
Hypertension (HTN)[1]
- Patients with mild-moderate HTN (defined by systolic blood pressure of 130-169 mmHg) have a hazard risk (HR) of 2.3 for SAH compared with patients with SBP <130.
- Patients with severe HTN (SBP >170 mmHg) have an HR of 3.3.
- The risk trend is similar for diastolic blood pressure.
Smoking[1]
- Former smokers have an HR of 2.7 compared with patients who have never smoked.
- Current smokers have an HR of 6.1 in the same comparison.
Cocaine Use[2]
- Cocaine use is associated with aneurysm risk in a younger population and in small vessels.
- Cocaine-related aneurysms have a higher mortality than others.
Non-modifiable Risks:
Age
- The incidence of SAH increases with age regardless of gender.[3]
Gender
- Females have an HR for rupture of 1.9 compared with males.[1]
Personal history of rupture
- As discussed in Part 1, history of SAH secondary to aneurysm rupture puts patients at a great risk of rupture (HR of 7.3 compared with no personal history of SAH).[4]
Genetic/Connective Tissue Disorders
- Autosomal dominant polycystic kidney disease
- Ehlers-Danlos Syndrome, Type IV (Vascular EDS)
- Marfan Syndrome
- Neurofibromatosis Type 1
- Pseudoxantoma Elasticum
- Loeys-Dietz Syndrome
- α1 –Antitrypsin Deficiency
Associated Conditions
- Fibromuscular Dysplasia (FMD)
- Arteriovenous Malformation
- Abdominal Aortic Aneurysm
- Sickle Cell Anemia
Family history of rupture
- In patients with polycystic kidney disease and at least one relative with aneurysms are 51 times more likely to have an aneurysm rupture compared with those who have no family history.[5]
Takeaways:
- We should counsel our patients heavily on the things that are within their control (the modifiable risks):
- Keep systolic blood pressure normotensive.
- Quit smoking if you are a current smoker or don’t start if you aren’t.
- Stay clear of cocaine.
- The non-modifiable risks are more useful to the provider — to help inform decision-making.
- You might consider screening patients for aneurysms if they have two primary relatives with known aneurysms (ruptured or unruptured) OR if they have one of the listed genetic conditions or associated conditions.
- Magnetic resonance angiogram (MRA) of the head is the preferred screening tool. Unlike computed tomography with angiography, MRA scans do not expose the patient to radiation and do not require a dye injection.
- Screening patients with some of the genetic disorders is controversial since their vessels tend to be more friable, making them more difficult and dangerous to treat.
The third and final part of this series will cover available treatment options for intracranial aneurysms.
Note: A special thanks to Dr. Thabele M. Leslie-Mazwi (neuroendovascular and neuro critical care specialist at Massachusetts General Hospital) for his help retrieving some of the studies used in this post.
References
- Sandvei, e.a., Risk Factors for Aneurysmal Subarachnoid Hemorrhage in a Prospective Population Study: The HUNT Study in Norway. Stroke, 2009. 40: p. 1958-1962.
- Nanda, A., et al, Intracranial Aneurysms and Cocaine Abuse: Analysis of Prognostic Indicators. Neurosurgery, 2000. 46(5): p. 1063-1069.
- N K de Rooij, e.a., Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry, 2007. 78: p. 1365-1372.
- Ishibashi, e.a., Unruptured Intracranial Aneurysms Incidence of Rupture and Risk Factors. Stroke, 2009. 40: p. 313-316.
- Irazabal, e.a., Extended Follow-Up of Unruptured Intracranial Aneurysms Detected by Presymptomatic Screening in Patients with Autosomal Dominant Polycystic Kidney Disease. Clin J Am Soc Nephrol, 2011. 6: p. 1274-1285.
October 26th, 2016
Friday Night Lights
Emily F. Moore, RN, MSN, CPNP-PC, CCRN
Most everyone has some association with football. Whether we know someone who’s played, played ourselves, or simply have nostalgic memories watching the game, football is a familiar sport to most Americans. I, for one, absolutely love football. Surprisingly, I never realized the danger involved with football until I met my husband, a high school football coach.
 Every time players suit up, they are making the conscious decision to put themselves at risk for injury. Similarly, by signing a release and allowing our children to play, we knowingly accept this risk and entrust the coach to protect and teach proper, safe technique. The severity of injuries can range from minimal to life threatening. A few weeks ago, my husband’s team endured seven injuries. A few boys sprained their ankle, one broke his collar bone, and one sustained a concussion from a hard hit. Few injuries keep players from returning to the game. The decision to return to the game is made by the coach, who largely relies on the player’s honesty. It has been documented, however, that many injuries, mainly concussions, are underreported for fear of being held from participation (Valovich McLeod et al 2007).
Every time players suit up, they are making the conscious decision to put themselves at risk for injury. Similarly, by signing a release and allowing our children to play, we knowingly accept this risk and entrust the coach to protect and teach proper, safe technique. The severity of injuries can range from minimal to life threatening. A few weeks ago, my husband’s team endured seven injuries. A few boys sprained their ankle, one broke his collar bone, and one sustained a concussion from a hard hit. Few injuries keep players from returning to the game. The decision to return to the game is made by the coach, who largely relies on the player’s honesty. It has been documented, however, that many injuries, mainly concussions, are underreported for fear of being held from participation (Valovich McLeod et al 2007).
What is the threshold we should set as parents in deciding when to keep our children from playing a particular sport? My husband regales me of stories from his childhood, when he would reenact and even “call plays” from his living room. His dream has always been to play and coach football. He even pursued a career just so he could coach high school ball. Knowing this, I ask, is it fair as parents to deprive our children of their dreams because of the risk?
It isn’t just football. According to recent statistics, soccer results in as many if not more head injuries than football (Gessel et al 2007). This is likely related to the larger number of youth (both boys and girls) who play soccer compared with football. Reportedly, the injuries sustained from soccer are generally related to players colliding into each other while simultaneously attempting to “head” the ball (Gessel). I distinctly remember, while working in the PICU, taking care of a teenager with a ruptured arteriovenous malformation from heading the soccer ball one too many times. Granted, the AVM was undiagnosed, but likely would not have ruptured without the repeated contact to the head.
As research continues to advance, more attention is placed on the danger of traumatic brain injury at a young age. We know that children ages 5-18 are much more vulnerable to head injuries than their adult counterparts. It is estimated that 30-45 million children participate in competitive sports. Additionally, there are approximately 200,000 head injuries seen in the emergency department each year (Gioia et al 2009). Traumatic brain injury is the leading case of hospitalization and death among children and adolescents and therefore represents a major public health problem (Langlois et al 2006). I ask myself, if enough parents decide they do not want their child to play any sport, whether it be football, soccer, hockey, or basketball, will that sport then shift to the margins? In other words, will risk outweigh the benefit and result in team sports slowly fading out of society? As we see more attention shifted to concussions and protection, one may question the future of competitive sports.
 As providers, it is our responsibility to provide appropriate anticipatory guidance to parents and players prior to signing a sports clearance. Prevention of injury focuses on education, not only to the athletic community but to the parents. So, are we doing enough, or should we be doing more? Is it our job to sit with each parent prior to signing a sports clearance and state the pros and cons of each sport? Do we explain what a brain injury looks like, what to remember when your child gets hit, and lastly, when to seek medical attention? Or do we assume that the current knowledge among coaches and parents within the athletic community is enough without the addition of the primary care provider’s input?
As providers, it is our responsibility to provide appropriate anticipatory guidance to parents and players prior to signing a sports clearance. Prevention of injury focuses on education, not only to the athletic community but to the parents. So, are we doing enough, or should we be doing more? Is it our job to sit with each parent prior to signing a sports clearance and state the pros and cons of each sport? Do we explain what a brain injury looks like, what to remember when your child gets hit, and lastly, when to seek medical attention? Or do we assume that the current knowledge among coaches and parents within the athletic community is enough without the addition of the primary care provider’s input?
On the other hand, there are many pros to competitive group sports. We are teaching our children how to be competitive but learn to lose at the same time — an invaluable life skill. We show our children how to help out their teammates and encourage those who are down. It teaches them how to work together as a team, with the common goal of winning. As a coach’s wife, I hear about the struggles and strengths of individual team members and have watched many football players overcome their struggles. I have also witnessed teammates encourage and build each other up during a game or in practice.
So, I ask my colleagues, is the benefit of sportsmanship worth the risk of a potentially life-changing injury? Or is that risk small enough that we can continue to look on and encourage the multitude of paybacks gained from team sports?
I personally feel that the life skills learned from competitive group sports are irreplaceable. However, the growing research regarding head injuries is concerning. Therefore, as a clinician I am torn. Moving forward, I will provide the proper counseling to my patients so I know that their decision to play or not was informed.
References:
Gessel, L. M., Fields, J.D., Collins, C. L., et al (2007). Concussions among United States high school and collegiate athletes. Journal of Athletic Training, 42(4), 495-503
Gioia, G. A., Schneider, J.C., Vaughan, C. G., et al (2009). Which symptom assessments and approaches are uniquely appropriate for paediatric concussion? British Journal of Sports Medicine, 43(suppl 1), i12-i22.
Langlois, J.A., Rutland-Brown, W., & Thomas, K.E. (2006). Traumatic Brain Injury in the Unites Sates: Emergency department visits, hospitalizations and deaths. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control.
Valovich McLeod, T.C., Schwartz, C., & Bay, R. C. (2007). Sports-related concussions misunderstanding among youth coaches, Clinical Journal of Sports Medicine, 2007(17), 140-141.
October 19th, 2016
Fighting Decision Fatigue
Harrison Reed, PA-C
It was an impossible choice. The seconds ticked by, but no matter how I strained my mind, I could not find a solution. She sighed and asked again.
“Harrison, where do you want to eat?”
I buried my face in my hands. How could this simple question stump a person who makes a hundred monumental choices each day, weighs a thousand variables, and carries the well-being of his patients on his shoulders? After ten hours of work without a proper meal, my hunger alone should have made it an automatic selection.
Our car made another lazy loop around the parking lot.
Tacos. Or ramen. Or Thai food? There’s sushi or burgers. Pizza is pretty bad for you. I already had a salad today, though. Wait, did I have a salad today? There’s that Greek place. How late is the barbecue restaurant open? I should buy groceries and cook. But what should I cook?
“I just… I just don’t know.” I held my head as if my liquefied brain threatened to spill out. Maybe this was my breaking point. Maybe I would starve, a life snuffed out by radical indecision.
“Just drive,” I said. “Anywhere.”
I didn’t know it at the time, but my gustatory conundrum was actually a symptom of decision fatigue. Like physical fatigue, decision fatigue is the gradual depletion of one’s power to make decisions through overuse. My inability to choose a meal after a shift at the hospital might seem trivial, but the principle has very real consequences.
The most famous example of high-stakes decision fatigue comes from a study that examined the decisions of parole boards at Israeli prisons. Researchers found that judges were far more likely to issue favorable decisions to prisoners seeking parole if the cases were heard early in the day. The likelihood of a favorable judgment declined with each subsequent case but improved after a break for food or rest. It seemed that for those seeking mercy from the court, timing was everything.
Decision fatigue plays a major role in medicine, too. Unlike the Israeli judges, we are not just making a handful of decisions before lunch. Our unique environment means that, in some cases, we might make dozens of decisions per patient at a pace we do not truly control. If even minor decisions can deplete mental reserve, the potential for complete decision exhaustion is astronomical.
It appears we do not need to be physically exhausted for our mental stamina to wane. That means decision fatigue can consume us without overt signs. Since consequences can be dire for patients seen late in a shift, it is paramount that we acknowledge the threat and monitor our state of mind. While some degree of decision fatigue is unavoidable, there are strategies that can reduce the burden.
- Delegate Decisions
If mental fatigue results from an accumulation of decisions, the best defense is to reduce the number of those decisions. I have the luxury of working with great nurses who spend more time at the bedside than I do and have extensive insight into patients’ conditions. If two choices are medically equal in my mind and without contraindications, I can defer the final decision to the patient’s nurse. Pharmacists, nutritionists, speech pathologists, and physical therapists (among many others) are also great resources to reduce decision burden. As long as the team has a unified plan, the path to achieve it may be a matter of style or preference.
- Decide and Commit
Once all necessary information is available, make the decision. If no new data are coming, putting off a decision will only increase the toll it takes on your mental reserve. Make a choice and stick to it knowing you can always reassess when new information is available.
- Plan in Advance
Have a contingency plan in place for likely outcomes or complications. Make your decision ahead of time—under less stress—so your plan is clear should an urgent need arise. If appropriate protocols exist at your institution, use them rather than trying to reinvent a solution on the fly.
- Take a Break
When a break from the action is possible, take the opportunity. Strong evidence suggests that being tired or hungry makes decisions harder. A break with or without a meal is likely to improve decision fatigue.
As with most problems, recognition and acknowledgement of decision fatigue is the first step in overcoming it. Discuss this issue with your team and formulate strategies to reduce it. Or don’t. The choice, of course, is yours.
Please share your experiences and solutions or tips to combat decision fatigue.
October 14th, 2016
From the Locker Room to the Exam Room
Elizabeth Donahue, RN, MSN, NP-C
On Sunday morning I went to an early and excruciatingly difficult barre class with a friend, after which we promptly rewarded ourselves with a decadent brunch. While walking home alone in the rain, hood drawn and head tucked down against the wind, I was taken aback when a man reached out and pushed me aside out of his path — doing so by placing his hand on my groin. Truly, it took me a few more strides to feel offended by the placement of his hand — much too personal a place to be used under the guise of avoiding a collision with a stranger. Offended was just the start though — I have spent the last several days stewing.
When someone in a position to influence national discourse (let alone domestic and foreign policy) dismisses conversations about touching women inappropriately as “locker room talk,” when an athlete is caught on tape abusing a partner and continues to play with only minor sanctions, or when a victim of sexual assault feels more vilified by the media than the perpetrator — we send a clear message to women that they are less than, that they are objects, that they do not deserve to control what happens to their bodies. This also sends a message to perpetrators of sexual violence that this behavior will be condoned, and I cannot begin to think of the message it sends children about gender roles, respect, equity, etc.
 Some have recently defended this type of discourse as “just words,” but unfortunately all too often talk leads to action. And in thinking about what happened on my walk home on Sunday, I began to feel sad — because the truth is I count myself among a lucky few women whose experience of degradation has been relatively limited, paling in comparison to the type of sexual assault others have known. I have borne witness to the effects of such experiences on my patients. The magnitude and gravity of those experiences stay with me.
Some have recently defended this type of discourse as “just words,” but unfortunately all too often talk leads to action. And in thinking about what happened on my walk home on Sunday, I began to feel sad — because the truth is I count myself among a lucky few women whose experience of degradation has been relatively limited, paling in comparison to the type of sexual assault others have known. I have borne witness to the effects of such experiences on my patients. The magnitude and gravity of those experiences stay with me.
I vividly remember the first time I was shocked by a story of domestic violence; it was within the first month of my practice. A woman in her mid 40s came in for a routine physical. She filled out a form indicating that yes, she felt she had been emotionally, verbally, or physically abused by someone in her household. When we asked if she would like to discuss it further, she shared a story of years of abuse at the hands of her husband — who had forced her repeatedly to engage in sexual intercourse with multiple partners while he looked on. She had suffered with depression and anxiety and had never sought care because she hadn’t felt safe to confess the source of her symptoms. Only when her husband had decided to ask for divorce did she find herself able to come forward and ask for help.
A couple of years later, a patient disclosed to me that she had been the patient of a gynecologist who had videotaped her Pap smears for years without her knowledge or consent. The gynecologist went on to be sued by several patients once the abuse was uncovered. Months after that I referred, for the first time, a patient to a gynecologist for a routine Pap smear to be performed under general anesthesia — she had been the victim of such brutal sexual assault that she could not obtain routine care without experiencing significant panic symptoms. Within the last week I have asked a social work colleague to participate in consults for three different woman in their 20s who have experienced post-traumatic stress disorder from sexual assaults that occurred in childhood.
Gender is considered a social determinant of health by the World Health Organization. Being a woman affects health outcomes in that it increases the possibility of violence, restricts access to resources, and correlates to a lower ability to participate in decision-making regarding health. It is reported that approximately 1.3 to 5.3 million women in the US experience intimate partner violence every year — ranging from psychological aggression to rape. Nearly 80% of female rape victims say they were first assaulted before age 25. And approximately 27% of women report that they experience short- or long-term health effects from sexual violence, including physical and mental health conditions — something I have seen over and over again in practice. Injuries reported around the world range from bruises to broken bones to loss of consciousness. Increased mental health diagnoses (such as depression and anxiety), substance use, and suicidal thoughts are also linked to violence experienced by women.
outcomes in that it increases the possibility of violence, restricts access to resources, and correlates to a lower ability to participate in decision-making regarding health. It is reported that approximately 1.3 to 5.3 million women in the US experience intimate partner violence every year — ranging from psychological aggression to rape. Nearly 80% of female rape victims say they were first assaulted before age 25. And approximately 27% of women report that they experience short- or long-term health effects from sexual violence, including physical and mental health conditions — something I have seen over and over again in practice. Injuries reported around the world range from bruises to broken bones to loss of consciousness. Increased mental health diagnoses (such as depression and anxiety), substance use, and suicidal thoughts are also linked to violence experienced by women.
A multi-country study done by the World Health Organization on women’s health and domestic violence against women explicitly stated that the “immediate social context” was a factor in rates of sexual abuse and could either protect women against or increase risk for violence. As providers, it is suggested that we create safe, non-judgmental spaces to provide support and treatment for women who have experienced violence. As a society, we need to change the social context — and it should start by no longer condoning the type of talk that suggests violence against women is acceptable. According to the Chinese proverb, “Women hold up half the sky” — and it’s time we hold them up, respecting them as equals.
Author’s note: This post is related to the specific correlation between sexual violence and effects on women’s health and does not seek to minimize the experience of any other group or individual experiences of sexual violence. It is well known that sexual violence does not discriminate — it has been recognized in all populations, regardless of gender identity, sexual orientation, income level, race or ethnicity. The roots of sexual violence should be examined and strategies must be in place to reduce violence across all populations.
October 12th, 2016
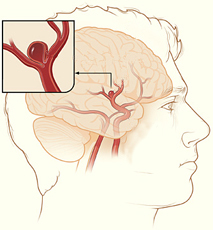
Intracranial Aneurysms for the Non-Neurosurgical Provider: Primer Series (Part 1)
Bianca Belcher, MPH, PA-C
Intracranial aneurysms are unnerving, but not all aneurysms are created equally. It’s no secret that nearly 50% of people with intracranial aneurysm ruptures die before they get to the hospital, and of the 50% who make it to the hospital, about 30% die despite our best efforts. Our office often gets urgent referrals from non-neurosurgical offices for incidentally found aneurysms that were discovered during workups for chief complaints such as dizziness or headache. Not all aneurysms are life threatening, and not all need an immediate referral to the emergency department; some just need monitoring by a provider who treats aneurysms on a regular basis.
 In this series of three primers on intracranial aneurysms, I will review the following: 1) the International Study of Unruptured Intracranial Aneurysms (ISUIA), 2) Patient Risk Factors, and 3) Treatment Options 101. The point of this series isn’t to create experts, but instead, to help non-neuro providers gain a better understanding of the neurosurgical thought process when considering next steps for these patients.
In this series of three primers on intracranial aneurysms, I will review the following: 1) the International Study of Unruptured Intracranial Aneurysms (ISUIA), 2) Patient Risk Factors, and 3) Treatment Options 101. The point of this series isn’t to create experts, but instead, to help non-neuro providers gain a better understanding of the neurosurgical thought process when considering next steps for these patients.
The International Study of Unruptured Intracranial Aneurysms (ISUIA) is probably one of the best natural history studies we have on aneurysms to date. There are two phases of ISUIA. Phase 1 (1998) was a two-part study: Part A, the retrospective arm, focused on the natural history of unruptured intracranial aneurysms. It looked at patients with and without history of subarachnoid hemorrhage (SAH) and determined if there were subgroups of people at elevated risk of rupture. In Part B, the prospective arm, the researchers primarily aimed to evaluate morbidity and mortality risks associated with treatment of unruptured aneurysms. Phase 2 (2003) was a prospective study that looked at the natural history of unruptured intracranial aneurysms as well as clinical outcomes and associated risks of aneurysm treatment, both endovascular and surgical. For the purposes of this blog, I will only focus on the natural history portions of each study (Phase 1, the retrospective arm, and Phase 2, natural history data).
Phase 1(1998) – ISUIA Retrospective Study
Overview:
- 53 centers (US, Canada, Europe)
- 1,449 patients, 1,937 aneurysms
- Group 1 (no history of SAH): 727
- Group 2 (history of SAH from a different aneurysm): 722
- Mean follow up: 8.3 years
- Aneurysm size groupings (<10 mm, 10–24 mm, ≥25 mm)
- Study strengths: large number of aneurysms, multi-center
- Study weaknesses: retrospective, potential for selection bias
Outcomes:
- 32 of 1,449 patients had confirmed aneurysm ruptures
- Group 1 ruptures (12/32): 11 in aneurysms ≥10 mm, 1 in aneurysm <10 mm
- Group 2 ruptures (20/32): 3 had aneurysms ≥10 mm, 17 had aneurysm <10 mm
- Relative risks of rupture by location of aneurysm (as compared to other locations)
- Group 1:
- 13.8 for basilar tip
- 13.6 for vertebrobasilar /posterior circulation
- 8.0 for posterior communicating (PCom) artery
- Group 2:
- 5.1 for basilar tip
- Group 1:
- Annual risk of rupture by size of aneurysm
- Group 1:
- <10 mm: 0.05%
- 10–24 mm: 1.0%
- ≥25 mm: 6.0%
- Group 2:
- <10 mm: 0.5%
- 10–24 mm: 1.0%
- ≥25 mm: not enough patients to calculate (n=3)
- Group 1:
- Significant predictors of rupture:
- Group 1: size and location of aneurysm
- Group 2: location of aneurysm and older patient age (RR, 1.31)
Phase 2 (2003) – ISUIA Prospective Study
Overview:
- 61 centers (US, Canada, Europe)
- 4,060 patients (1,692 no intervention [natural history], 2,368 underwent treatment)
- 2,686 aneurysms
- Group 1 (natural history cohort; no history of SAH): 1,077
- Group 2 (natural history cohort; history of SAH from a different aneurysm): 615
- Mean follow-up: 4.1 years
- Study strengths: prospective, multi-center, large number of aneurysms
- Study weaknesses: non-randomized, short follow-up period
Outcomes:
- 51 of 1,692 patients had confirmed aneurysm ruptures
- Group 1 ruptures (41/51): 2 aneurysms <7 mm, 5 aneurysms 7–9 mm
- Group 2 ruptures (10/51): 8 aneurysms <10 mm, 2 aneurysms ≥10 mm
- Cavernous carotid artery and anterior circulation aneurysms <7 mm in those patients without a history of SAH had a 0% risk of rupture.
- Cavernous carotid artery aneurysms <13 mm, regardless of history of SAH, had a 0% risk of rupture.
- Anterior circulation and posterior circulation aneurysms 7–12 mm had 5-year cumulative rupture rates of 3% and 15%, respectively.
Another relevant study:
One of the more recent studies being discussed in the cerebrovascular care community is out of Japan, The Unruptured Cerebral Aneurysm Study of Japan (UCAS Japan) 2012 (5,720 patients, 6,697 aneurysms). Unlike the ISUIA Phase 2, UCAS authors assessed an annual rupture rate (0.95%) as opposed to a 5-year rate and considered treatment for aneurysms that are ≥5 mm, as opposed to ≥7 mm in ISUIA. In another difference, UCAS authors found that non-smooth aneurysms (those with daughter domes) have a higher rupture rate compared with smooth aneurysms. Lastly, with regard to location, UCAS results show higher risk of rupture in both the ACom and PCom regions, whereas ISUIA identifies higher rupture rates only in the posterior circulation. The downside to this study is its limited generalizability outside of the Japanese population, who have a higher risk for subarachnoid hemorrhage than other populations.
Key Takeaways:
- ISUIA offers a good place to start the “treat or monitor” discussion. Although it’s an older study, ISUIA is probably one of the best/largest studies with respect to natural history of aneurysms and one that we quote to patients frequently. Don’t get me wrong; it’s not a perfect study. There are criticisms of the study design and likely some selection bias involved, but both phases of ISUIA still hold value.
- If you were to consider only aneurysm size/location/prior history of rupture, aneurysm treatment is considered if one or more of the following circumstances is met:
- Size is ≥5 mm
- Location in the posterior circulation or ACom artery
- Patient has a prior history of cerebral aneurysm rupture
- Aneurysm has an odd, non-smooth morphology (daughter domes)
- Aneurysm changes in shape or size between interval imaging
- Patients with a prior history of SAH are 10 times more likely to have rupture (of aneurysms <10 mm) than a patient without a history of SAH, though it is important to note that the annual rupture rate still remains relatively low in patients with a prior history at 0.5% per year.
- Cavernous aneurysms rarely rupture unless they are giant. Important note: True cavernous aneurysms are not located in the dura. They are located in the cavernous sinus, meaning that if they rupture, they do not cause SAH and are unlikely to kill your patient. Ruptures present as carotid-cavernous fistulas (CCF) with symptoms such as exophthalmos, ocular motor palsy (3rd or 6th depending on the directionality), and vision problems. If you suspect a cavernous aneurysm rupture, the patient needs to be seen by a cerebrovascular surgeon/neurointerventionalist quickly. Any cranial nerve symptoms that are secondary to the CCF can become permanent if it is not treated soon after rupture.
In Part 2, I will present modifiable and non-modifiable patient risk factors with regard to intracranial aneurysm treatment considerations.
References
The International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: Risk of rupture and risks of surgical intervention. N Engl J Med 1998; 339:1725.
Wiebers DO et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003; 362:103.
The UCAS Japan Investigators. The Unruptured Cerebral Aneurysm Study of Japan (UCAS Japan). N Engl J Med 2012; 366:2474.
September 29th, 2016
Why I No Longer Tolerate Anti-Vaxxers
Emily F. Moore, RN, MSN, CPNP-PC, CCRN
One may ask why I am all of a sudden fixated on vaccines. I have always been a pro-vaxxer. In graduate school, I once prided myself for talking a whole family into getting vaccines. And I certainly have always promoted vaccines when practicing.
But I used to think that the decision to vaccinate, although important, was not mine to make. Parents have their own reasoning, and my job as the clinician was to provide proper education but then support the decision made. Of course, vaccinating my own children was a no-brainer. (Why wouldn’t I choose to protect them from terrible diseases?)
Then my two-year-old was diagnosed with pertussis. The days following were a blur. I was 38 weeks pregnant and desperately trying to get my daughter the help she needed while also praying that my unborn baby didn’t make an early debut. I had always thought that my own children would be protected because they were vaccinated, and if for whatever reason something happened, the high percentage of vaccinated children would provide extra security.

Here is what happened. My daughter was coughed on while playing at the park. I shrugged it off; kids get sick, and we can’t protect them from everything. About a week later, she developed a terrible cough followed by a high fever. As the days progressed, she developed posttussive emesis and a cough that would stop anyone in their tracks. In the back of my mind, I thought, oh my gosh, she has whooping cough. How crazy would that be though? She’s fully vaccinated. So I chalked it up to a cold and ignored it.
Because I was busy getting my hours in before maternity leave, I had my husband take her to our pediatrician. Our pediatrician called me at work and more or less reassured me that pertussis was not within the differential. She said that our daughter had a bad cough and needed time to recover. I reminded her that I was 12 days away from having a baby and wanted to make sure all was well. So, we made a plan; if the cough wasn’t better in the next 5 days, we would test her.
Well, we didn’t make it 5 days. One day later in the middle of the night, the most heart-stopping cough woke me up. I ran to my daughter’s room and found her gasping (whooping) for air with intermittent bouts of apnea. I yelled to my husband to call 911. By the time he deciphered what I was yelling, my daughter had recovered. She slept with us that night and had two more episodes. The next day we went to urgent care. There, I was belittled and told that there was no way my vaccinated child had pertussis. I mean, after all, she looked perfect in the exam room (doesn’t this always happen?). I must not recognize what croup looks like in a “non-cardiac” baby (cardiology being my practice specialty). To say that this infuriated me is an understatement. Regardless, I was determined to get a diagnosis.
What finally convinced the urgent care providers to send a culture was my advanced pregnancy. However, this, too, was followed with patronizing comments. They wouldn’t even discuss treatment options, stating that they were 99.9% certain that she had croup. I left the appointment thinking I must be crazy.
Later that day I got the phone call — my daughter had pertussis.
A positive culture turned our worlds upside down. We were reported to public health, I couldn’t return to work, my daughter couldn’t leave the house, and everyone in our family needed to be treated. On top of that, the cough continued for weeks. They say pertussis is the 100-day cough, and I can see why.
My daughter catching pertussis, a preventable disease, has been one of the scariest experiences I have had as a parent. Watching her suffer from an absolutely paralyzing cough with apnea was indescribably horrible. This experience taught me two things: First, always listen to parents. They truly know their children best. Second, the decision to vaccinate should not be taken lightly. I will no longer sit quietly when caring for unvaccinated children. I will voice my bias. I once thought I didn’t support anti-vaxxers. Now I can state from experience: I do not tolerate them. If given the choice, I will not treat patients whose parents choose not to vaccinate them.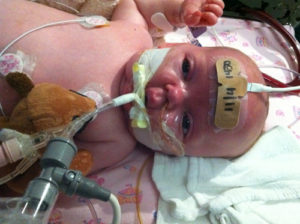
September 21st, 2016
An American in Yorkshire: Initial Impressions of the NHS
Megan Tetlow, PA-C

Megan Tetlow, PA-C, is from Fort Myers, Florida, now working in Sheffield, England, as part of the National Physician Associate Expansion Program. She practices in gynecologic oncology and is a guest blogger for In Practice.
Faster than you can say “cheers,” summer is turning into fall, and I find myself having passed the 2-month mark as a physician assistant working for the National Health Service (NHS) in Yorkshire, England, as part of the National Physician Associate Expansion Program (NPAEP). There have been learning experiences, celebrations, crossed wires, and countless cups of tea as I continue to learn more about the NHS and the role for PAs here in the U.K. Today, I’d like to share a couple of misconceptions I had about the NHS prior to making the journey to jolly old England.
Waiting Times
Since the U.K. has nationalized healthcare, I had envisioned months-long waiting lists for appointments and surgeries, particularly to see a specialist. While I cannot speak for other areas in medicine, in gynecologic oncology this has not at all been the case. The reason why,  I believe, has a lot to do with the target-date-based pathway system the NHS uses, which prioritizes patients with a diagnosis of a gynecologic malignancy. With a cancer diagnosis, a window is assigned in which the patient must be seen by the appropriate specialist and receive definitive treatment (31 and 62 days, respectively). Additionally, there are special “fast-track” clinics that I work in that exclusively deal with patients presenting with symptoms suspicious of a gynecologic cancer, and these patients must be seen within 2 weeks. I think you would be hard-pressed to consistently meet that mark when referring to a specialist in the U.S. Additionally, there are sanctions if not enough patients are seen and treated within the specified windows. So understandably, there is a focus to get these patients seen as soon as possible. And in working terms, it means that patients are seen by the diagnostic gynecology team (which includes yours truly) and, if needed, the gynecologic oncology team (which as it happens, also includes me) pretty impressively quickly.
I believe, has a lot to do with the target-date-based pathway system the NHS uses, which prioritizes patients with a diagnosis of a gynecologic malignancy. With a cancer diagnosis, a window is assigned in which the patient must be seen by the appropriate specialist and receive definitive treatment (31 and 62 days, respectively). Additionally, there are special “fast-track” clinics that I work in that exclusively deal with patients presenting with symptoms suspicious of a gynecologic cancer, and these patients must be seen within 2 weeks. I think you would be hard-pressed to consistently meet that mark when referring to a specialist in the U.S. Additionally, there are sanctions if not enough patients are seen and treated within the specified windows. So understandably, there is a focus to get these patients seen as soon as possible. And in working terms, it means that patients are seen by the diagnostic gynecology team (which includes yours truly) and, if needed, the gynecologic oncology team (which as it happens, also includes me) pretty impressively quickly.
Team-Based Approach
 Another misconception I had about working in the NHS is that doctors and patients would be resistant to the idea of physician assistants. This again has not at all been my experience. Doctors in the NHS seem very interested in my role, training, and the type of work I’m able to perform. As in the U.S., the NHS is experiencing a shortage of healthcare providers. My experience is that most doctors are very open to midlevel providers and other solutions that would help meet some of that patient demand. Furthermore, since every hospital in the U.K. trains physicians (as opposed to solely academic centers doing the training, like in the U.S.), these physicians-in-training make up a large part of the care providers in the NHS. I believe that as a result of this, doctors, staff, and patients especially all seem quite comfortable with the idea of team-based healthcare. Coming from the U.S., I was accustomed to explaining my role as a physician assistant to new patients and envisioned I would be having these discussions a lot more in the U.K. But what I’ve found is that patients are so used to seeing a physician-in-training or advanced nurse specialist when they have an appointment with an attending physician, that when Megan the PA walks in instead, it seems not to faze them in the least. (This does not extend to my accent, which is often a topic of conversation, and patients seem to enjoy betting their significant other over whether I’m American or Canadian.) Overall, patients seem happy to be seen and provided good care, regardless of my specific role (or accent for that matter).
Another misconception I had about working in the NHS is that doctors and patients would be resistant to the idea of physician assistants. This again has not at all been my experience. Doctors in the NHS seem very interested in my role, training, and the type of work I’m able to perform. As in the U.S., the NHS is experiencing a shortage of healthcare providers. My experience is that most doctors are very open to midlevel providers and other solutions that would help meet some of that patient demand. Furthermore, since every hospital in the U.K. trains physicians (as opposed to solely academic centers doing the training, like in the U.S.), these physicians-in-training make up a large part of the care providers in the NHS. I believe that as a result of this, doctors, staff, and patients especially all seem quite comfortable with the idea of team-based healthcare. Coming from the U.S., I was accustomed to explaining my role as a physician assistant to new patients and envisioned I would be having these discussions a lot more in the U.K. But what I’ve found is that patients are so used to seeing a physician-in-training or advanced nurse specialist when they have an appointment with an attending physician, that when Megan the PA walks in instead, it seems not to faze them in the least. (This does not extend to my accent, which is often a topic of conversation, and patients seem to enjoy betting their significant other over whether I’m American or Canadian.) Overall, patients seem happy to be seen and provided good care, regardless of my specific role (or accent for that matter).
These are just a couple of the comparisons between the American and British healthcare systems that I’ve been attempting to mentally catalogue during these past months. This experience continues to be eye-opening, exciting, and at times a bit daunting. I look forward to exploring and dissecting more of these similarities and differences in the near future. Until then, if you have any questions about the workings of the NHS or physician associates in the U.K., please post them below and I will try to address them in a future post.