January 13th, 2014
Merck’s Vorapaxar Gets Positive FDA Review
Larry Husten, PHD
A few years ago a novel antiplatelet agent from Merck seemed all but dead. Vorapaxar, a thrombin receptor antagonist, was widely thought to have no future after unacceptably high serious bleeding rates were found in two large clinical trials studying the drug in a wide variety of acute and chronic cardiovascular patients. But hopes for the drug resurfaced with a new analysis of one of those trials, the TRA2P trial. Now the FDA appears willing to give the drug a renewed lease on life.
On Wednesday the Cardiovascular and Renal Drugs Advisory Committee will discuss the new drug application (NDA) for vorapaxar (proposed trade name, Zontivity) for a considerably narrower indication than initially hoped for by the company. The proposed indication is as an adjunct therapy for the reduction of atherothrombotic events in patients with a history of myocardial infarction (MI). The company also wants to claim the drug leads to a reduction of the combined endpoint of cardiovascular death, MI, stroke, and urgent coronary revascularization.
In the full TRA2P trial, 26,449 patients with a history of MI, ischemic stroke, or peripheral arterial disease were randomized to either vorapaxar or placebo in addition to standard therapy. Vorapaxar was found to be effective in cutting the rate of CV death, MI, or stroke. But it was also associated with a doubling of the very serious complication of intracranial bleeding. This finding, along with the high rate of bleeding complications and intracranial hemorrhage that occurred in the TRACER trial in acute coronary syndrome patients, seemed likely to doom the drug.
But TRA2P was redesigned in midcourse based on Data and Safety Monitoring Board concerns about patients with a history of stroke. Patients with stroke were no longer allowed to enter the trial. In the subgroup analysis of the 17,779 patients with a history of MI and no history of stroke, there was a significant reduction in cardiovascular events with no increase in intracranial bleeding.
In general, a substudy does not provide sufficient evidence to support an NDA for a novel drug. But the TRA2P substudy was larger than is usually found in the entire population of most clinical trials. The FDA reviewers conclude that the “results are sufficient to establish the effectiveness of vorapaxar for its proposed indication in patients with prior MI and support the Applicant’s proposal not to include patients with prior stroke in the target population.”
The FDA will webcast the entire session on Wednesday. (Click here for webcast information.)
January 10th, 2014
ESC Spotlights Growing Problem of Radiation Exposure in Cardiology
Larry Husten, PHD
Both medical professionals and patients have a general sense that radiation used in medical imaging and procedures carries some danger, but they often underestimate the risk. And certainly most are unaware of the increasingly large proportion of the problem occurring during cardiology procedures.
“Cardiologists today are the true contemporary radiologists,” said Eugenio Picano, lead author of a report from the European Society of Cardiology published in the European Heart Journal. “Cardiology accounts for 40% of patient radiology exposure and equals more than 50 chest X-rays per person per year,” he said in a press release. And cardiologists themselves can be exposed to radiation at two to three times the levels of diagnostic radiologists, according to the report.
The major barrier to recognizing the problem, of course, is that it may take decades before cancers emerge as a result of the radiation. Further, the authors note, “radiation-induced cancer is clinically undistinguishable from a spontaneously occurring cancer.”
Many patients are exposed to high doses of radiation (>50 mSv) for which there is epidemiological evidence showing a link to cancer. The authors estimate that patients exposed to 100 mSv, which is equivalent to about 5000 chest x-rays, will have “an additional risk as high as 1 in 30 or as low as 1 in 300.”
Nuclear cardiologists can receive as much as 2-5 mSv a year while interventional cardiologists and electrophysiologists have an annual exposure of about 5 mSv, which is two to three times higher than the exposure of diagnostic radiologists. This exposure, they calculate, results in “a typical cumulative lifetime attributable risk on the order of magnitude of 1 cancer (fatal and non-fatal) per 100 exposed subjects.”
Children, of course, are at greatest risk, “because they have more rapidly dividing cells and a greater life expectancy.”
“Even in the best centers, and even when the income of doctors is not related to number of examinations performed, 30 to 50% of examinations are totally or partially inappropriate according to specialty recommendations,” said Picano, in the press release. “When examinations are appropriate, the dose is often not systematically audited and therefore not optimized, with values which are 2 to 10 times higher than the reference, expected dose.”
January 9th, 2014
Pivotal Renal Denervation Trial Fails to Show Efficacy
Larry Husten, PHD
Medtronic announced today that the SYMPLICITY HTN-3 trial of its much-anticipated renal denervation device had failed to meet its primary efficacy endpoint. Renal denervation has been widely touted as a breakthrough product that could dramatically lower blood pressure by as much as 30 mm Hg, allowing physicians to cure the most severe form of high blood pressure, resistant hypertension.
“SYMPLICITY HTN-3 met its primary safety endpoint related to the incidence of major adverse events one month following randomization and renal artery stenosis to six months,” said Deepak Bhatt, the co-principal investigator of the trial, in the Medtronic press release. “Importantly, however, the trial did not meet its primary efficacy endpoint.” To demonstrate efficacy in the trial, blood pressure in the treatment arm would have needed to be 10 mm Hg lower than in the control arm.
Medtronic said that because no safety concerns emerged in the trial, “no specific action is currently indicated for patients who have had the renal denervation procedure with the Symplicity system.” The company said it will assemble a panel of independent advisors “to make recommendations about the future of the global hypertension clinical trial program.” Until then Medtronic said it would suspend enrollment in ongoing trials, including the SYMPLICITY HTN-4 trial studying renal denervation in patients with less severe forms of hypertension. Symplicity will still be available in Europe and other markets where it has been approved and the company will continue its global post-market surveillance registry as well as studies that are evaluating other non-hypertension indications for the device.
Wells Fargo analyst Larry Biegelsen said that the news means that that Medtronic is unlikely to gain U.S. approval for the device without first running another trial.
Until recently the forecast for renal denervation had been extremely optimistic. More realistic expectations began to appear in 2013. Last summer, a paper published in Heart showed that the large reductions in blood pressure seen in earlier clinical trials of renal denervation were likely a product of serious flaws in the design of these trials. Until SYMPLICITY HTN-3, the renal denervation trials had been largely uncontrolled, unblinded, and had utilized office-based blood pressure measurements rather than the far more reliable and consistent ambulatory blood pressure monitoring. The senior author of the Heart paper, Darrel Francis, predicted that “people are going to be severely disappointed” by the results of SYMPLICITY HTN-3.
In December, St. Jude confirmed that it had halted enrollment in its EnligHTN IV trial, which was the pivotal trial for its own renal-denervation device. Renal-denervation products are currently available in Europe and elsewhere from Medtronic, St. Jude Medical, Boston Scientific, Covidien, Recor, and Terumo.
January 9th, 2014
Hospital Quality Helps Explain Some Racial Disparities in CABG Outcomes
Larry Husten, PHD
It has long been known that racial disparities exist in health care. A large body of research has found that nonwhite patients have worse outcomes than whites. But it has been difficult to understand the underlying reasons for these disparities. Now a new study offers evidence that, at least in the case of bypass surgery, a significant but by no means complete portion of this disparity is due to decreased access among nonwhites to high-quality hospitals.
In a paper published in JAMA Surgery, Govind Rangrass and colleagues analyzed Medicare data from 173,925 patients undergoing CABG. Of these patients, 8.6% were nonwhite. The mortality rate was 3.6% for the entire population. Nonwhite patients had a 34% increased risk of dying.
A key finding was that the third of hospitals that had the highest proportion of nonwhite patients (more than 17.7% nonwhite) also had the highest risk-adjusted mortality for both white and nonwhite patients (3.8% and 4.8%). In sharp contrast, the third of hospitals treating the fewest number of nonwhite patients (less than 2%) had the lowest risk-adjusted mortality for both white and nonwhite patients (3.2% and 3.7%). So at the best hospitals nonwhite patients did about as well as the white patients at the worst hospitals.
The investigators then adjusted for other patient factors and found that nonwhite patients were still at increased risk, with a 33% higher risk of death (odds ratio 1.33, CI 1.23-1.45). When patient factors were considered along with socioeconomic status and hospital quality, the investigators reported that they could then account for 53% of the racial disparity.
The authors speculated that the remaining unexplained disparity might be due to differences in disease severity: nonwhites may seek care at later or more severe stages of disease. Other possible factors mentioned are regional variations in the quality of hospitals, proximity to good hospitals, and segregated referral patterns.
They propose that “more should be done at a systems level to bring higher-quality care to disadvantaged populations,” but acknowledge that evidence-based policy solutions are lacking. “With a better understanding of the barriers to high-quality care, we will be able to design more effective programs to decrease health disparities,” they conclude.
January 8th, 2014
Women in Cardiology: The Challenge to Have Both Career and Family
Monika Sanghavi, MD
Monika Sanghavi’s recent Circulation: Cardiovascular Quality and Outcomes article tackles the important topic of the under-representation of women in cardiology and the reasons behind it, particularly the challenge of balancing a demanding career with family. CardioExchange asked Monika to share why she wrote the blog and the reaction she’s received.
The book Lean In was released on March 11th 2013, the last day of ACC 2013. As part of the book promotion, Katie Couric had interviewed Sheryl Sandberg on her talk show and tweeted a statement from Sandberg: “Women should go for the big job and deal with family later.” I immediately tweeted back, “I agree women should strive for greatness, but at the expense of their family, not sure.”
After I sent the tweet, I had an instant realization that I was being a hypocrite. I had been living apart from my husband for two years during fellowship in order to pursue my career ambitions. If that isn’t deferring family obligations, what is? I considered that many would think that living apart from their spouse was too high of an opportunity cost to pursue a cardiology fellowship. I reflected on this and the other sacrifices we make in order to pursue our passion in medicine.
While at the ACC sessions, I began to notice how most of the panelists of the late-breaking trials were men and how the conference was attended by more men than women. I also reflected on the fellows and faculty in my division and realized that women were disproportionately affected by family responsibilities: compared with our male counterparts, more of the female fellows had deferred starting a family and more of the female faculty had taken part-time positions to balance their family obligations.
I began to wonder if this is why we have such an under-representation of women in cardiology and what could be done to level the playing field for women. It is obviously not the hard work, overnight call, or subject matter that deters women, or there wouldn’t be so many in General Surgery, OBGyn, and Pediatric Cardiology.
When I flew home that night I couldn’t sleep; I wrote all of my thoughts on paper and began discussing this topic with other people. My informal survey of the women faculty at my institution reflects the findings of the ACC Professional Life Survey. The struggles these women were facing were not isolated, but part of a national struggle.
With the encouragement of my co-fellows and faculty mentors, I decided to publish my thoughts. The response has been great. I received an e-mail from a resident applying to cardiology in which she said, “You have captured my fears exactly.” I’ve also gotten emails from more senior faculty of both genders who appreciate the discussion. I really hope this article helps others reflect on this important issue and is a catalyst for greater discussion on what can be done to help recruit women into the field of cardiology and to balance both their career goals and family responsibilities.
January 7th, 2014
Is It Time to Sunset the Holter Monitor?
Paddy Barrett, MB BCh BAO MRCPI MCTI
CardioExchange’s John Ryan interviews Paddy M. Barrett about his research group’s study, published in the American Journal of Medicine, comparing 24-hour Holter monitoring with the Zio Patch 14-day single-lead ECG device.
THE STUDY
One hundred forty-six patients who had been referred for evaluation of cardiac arrhythmias underwent simultaneous ambulatory ECG recording with a conventional 24-hour Holter monitor and with the single-lead Zio Patch 14-day adhesive patch monitor. Over the total wear time of both devices, the adhesive patch monitor detected significantly more arrhythmia events than the Holter monitor did (96 vs. 61). During the initial, simultaneous 24-hour monitoring period for the two devices, the Zio Patch monitor detected significantly fewer events than the Holter monitor (52 vs. 61). The maker of Zio Patch partly funded the study.
THE INTERVIEW
Ryan: Why did you compare a 24-hour device (Holter monitor) with a 14-day device (Zio Patch)? Did that affect your results?
Barrett: The most commonly used platform for detecting cardiac arrhythmias is the Holter monitor, which (with some modifications) has been used since the 1960s. Typically, Holter monitoring is conducted for 24 hours, as in our study. With the explosion in novel biosensors, we felt that a direct comparison of the Holter monitor, as it is traditionally used, with a novel 14-day patch sensor was a valuable question to answer in assessing incremental benefits in arrhythmia detection.
Ryan: Why do you think the Zio device missed some events in the first 24 hours? If a patient agrees to only 24 hours of monitoring, can Zio be recommended for this purpose, given the evidence to date?
Barrett: We believe the Zio Patch can be used for any duration up to at least 14 days. A crucial factor, of course, is the potential frequency of arrhythmias in the monitored patients, some of whom will be asymptomatic (the longer the monitoring period, the better).
The primary reason that Zio Patch did not detect some arrhythmia events in the first 24 hours was that our study defined events by very short episodes, often only four sequential beats. The Zio Patch detected these, but because the raw data were initially screened by an iRhythm physician who prepared the report, some of those very short episodes were not included in the report. iRhythm now populates all arrhythmia events to the report.
Ryan: What was the rate of allergic reactions in the study? Will chest hair, adiposity and emphysema interfere with detection of events?
Barrett: Allergic reactions were very infrequent. This has been a major area of focus in this area, as high rates of skin allergy in patients have been documented for historical devices. Even up to 14 days, we had very few skin reactions. A small section of chest hair was shaved to apply the device, but we did not find that additional adiposity or larger lung volumes impeded arrhythmia detection.
Ryan: When can providers start prescribing the Zio device — and how?
Barrett: The Zio Patch is available right now and can be prescribed in two ways. First, the physician can purchase a stock and apply the device in the office; the patient returns the device to iRhythm by mail for analysis and generation of a report, which is made available to the ordering physician. Second, the physician can have the device independently mailed to the patient, who then self-applies it.
Ryan: Is it realistic that the Zio device will replace the Holter monitor any time in the foreseeable future?
Barrett: Absolutely — this device and others like it will replace the Holter monitor. Using a device the size of a VHS cassette with multiple wires and stickers is just not a suitable method for detecting arrhythmia events when novel biosensor platforms exist (and will evolve even further). It is not a technology of the future; it is a technology of today.
Given Dr. Barrett’s analysis, what do you think the future of arrhythmia-detection devices like Zio Patch will be?
January 6th, 2014
Mediterranean Diet Protects Against Diabetes, Regardless of Weight Loss
Larry Husten, PHD
Even if it doesn’t lead to weight loss, a Mediterranean diet could help prevent the onset of type 2 diabetes, according to a subanalysis of last year’s influential PREDIMED study. In the main trial, reported in the New England Journal of Medicine, nearly 7500 people at high risk for cardiovascular disease were randomized to a low-fat diet or a Mediterranean diet supplemented by either extra-virgin olive oil (EVOO) or nuts. After nearly 5 years’ follow-up, the study was stopped early because of a significant reduction in cardiovascular events in the Mediterranean diet groups.
The new paper, published in the Annals of Internal Medicine, examines the development of diabetes — a prespecified secondary outcome — among the 3541 participants who did not have diabetes at baseline and for whom the follow-up diabetes status was available. After 4.1 years’ follow-up, there was a significant, 30% reduction in the risk for diabetes in the combined Mediterranean diet groups compared with the low-fat diet group (HR 0.70, CI 0.54 – 0.92). Separately, the reduction was significant in the EVOO group but not in the nuts group. New-onset diabetes had occurred in 6.9% of people in the EVOO group, 7.4% in the nuts group, and 8.8% in the control group.
The differences in outcome appeared to be unrelated to weight loss, as the differences in weight loss across the groups were “negligible.” The authors explain that the major goal of the trial was “to change the overall dietary pattern,” and they did not attempt to reduce calories or increase physical activity.
It should be noted that more patients were lost to follow-up in the control group than in the Mediterranean diet groups (10.5% in the control group versus 6.9% in the nuts group and 4.1% in the EVOO group). The authors warn that any conclusions must be considered exploratory given that this was a substudy based on a subgroup of a larger trial.
Nevertheless, they conclude that PREDIMED “provides strong evidence that long-term adherence to a Mediterranean diet supplemented with EVOO without energy restrictions, which is high in monounsaturated fat and bioactive polyphenols, results in a substantial reduction in the risk for type 2 diabetes among older persons with high cardiovascular risk. Of note, this dietary pattern is palatable and has a high potential for long-term sustainability, with obvious public health implications for primary prevention of diabetes.”
January 6th, 2014
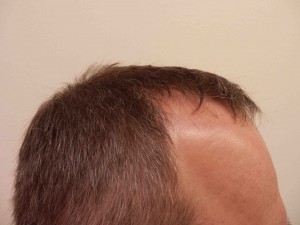
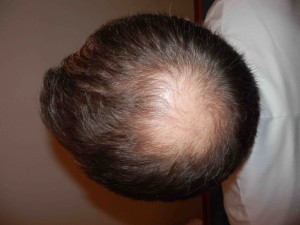
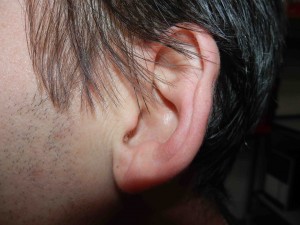
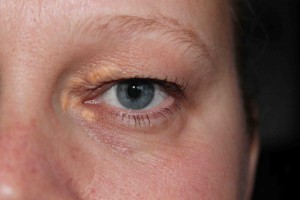
Looking Old for Your Age
Mette Christoffersen, MSc (Pharm)
A recent paper published in Circulation concluded that visible age-related signs — male pattern baldness, earlobe crease, and xanthelasmata — were associated with increased risk of coronary disease independent of chronological age and other cardiovascular risk factors. Study authors Mette Christoffersen and Anne Tybjaerg-Hansen answer questions from CardioExchange Editor-in-Chief Harlan Krumholz about their findings.
Krumholz: Your findings are quite interesting. Do you think that these findings should be sought on every patient?
Christoffersen and Tybjaerg-Hansen: Yes, this is very easy information to obtain. The data show that patients who look older than their chronological age based on these signs are more likely to be in poor cardiovascular health, compared with patients who appear to be their actual age.
Krumholz: Do you think that these findings should be part of cardiovascular risk calculators?
Christoffersen and Tybjaerg-Hansen: No, probably not. However, if you have one or more of these signs, other risk factors for cardiovascular disease should be evaluated carefully.
Krumholz: What do you think is the mechanism of this association?
Christoffersen and Tybjaerg-Hansen: This is not known, therefore, the following information is speculation: Regarding male pattern baldness and vascular disease, free testosterone has been shown to act both on the hair follicle and on the vascular wall, leading to both male pattern baldness and proliferation of smooth muscle cells — a key event in the formation of the atherosclerotic plaque.
Possible explanations for the increased risk of ischemic heart disease associated with the presence of earlobe crease and xanthelasmata may be altered characteristics of the connective tissue in these individuals reflected both in the dermis (skin) and in the arterial intima (vessel wall) — i.e., an increased propensity to deposit cholesterol in these tissues. The study shows that the presence of xanthelasmata is not explained by the high cholesterol alone.
January 6th, 2014
Selections from Richard Lehman’s Literature Review: January 6th
Richard Lehman, BM, BCh, MRCGP
CardioExchange is pleased to reprint this selection from Dr. Richard Lehman’s weekly journal review blog at BMJ.com. Selected summaries are relevant to our audience, but we encourage members to engage with the entire blog.
NEJM 2 Jan 2014 Vol 370
Stenting and Medical Therapy for Atherosclerotic Renal-Artery Stenosis (pg. 13): There’s scale in the pipes: call for Mr Stent the plumber. This has proved an enormously appealing idea for patients and plumbers over the last two decades, and Messrs Stent and Sons now have an annual turnover of billions. Pushing mesh into arteries is a rite of passage in many specialties, and even patients often talk of their stents with a kind of pride. A shame that most of them do nothing at all, even in coronary arteries. In renal arteries they may be universally pointless, according to this large publicly funded American trial. ” We randomly assigned 947 participants who had atherosclerotic renal-artery stenosis and either systolic hypertension while taking two or more antihypertensive drugs or chronic kidney disease to medical therapy plus renal-artery stenting or medical therapy alone. Renal-artery stenting did not confer a significant benefit with respect to the prevention of clinical events when added to comprehensive, multifactorial medical therapy in people with atherosclerotic renal-artery stenosis and hypertension or chronic kidney disease.”
Mitral-Valve Repair vs. Replacement for Severe Ischemic Mitral Regurgitation (pg. 23): If you suffer major ischaemic damage to your left ventricle, it is quite likely to fibrose and remodel itself in such a way as to distort the mitral valve and cause what is known as “functional” mitral regurgitation. This is associated with a substantial increase in the risk of progressive heart failure and death, and it affects a lot of people—probably over two million in the US alone. The leaflets of the mitral valve remain healthy, but their alignment is wrong, so if surgical treatment is attempted, it is usually in the form of mitral valve repair rather than replacement. But that may not be as good in the long term as replacing the valve, according to the authors of a trial which randomly assigned 251 patients with severe ischemic mitral regurgitation to undergo either mitral valve repair or chordal-sparing replacement. In the short term, clinical outcomes are identical, but in the long term, valve replacement is likely to prove more durable.
Unexpected Abrupt Increase in Left Ventricular Assist Device Thrombosis (pg. 33): Left ventricular assist devices have become popular in America, where a brand leader is HeartMate II, a small axial-flow device made by Thoratec. Unfortunately there seems to have been an epidemic of pump-related thrombosis in these devices associated with high morbidity and mortality, which began abruptly in March 2011. It shows no sign of stopping.
JAMA 1 Jan 2014 Vol 311
Effect of Prehospital Induction of Mild Hypothermia on Survival and Neurological Status Among Adults With Cardiac Arrest (pg. 45): Another trial shows that cooling people down on the way to hospital following cardiac arrest in the community makes no difference to outcomes.
Mechanical Chest Compressions and Simultaneous Defibrillation vs. Conventional CPR in Out-of-Hospital Cardiac Arrest (pg. 53): Nor does mechanically assisted cardiopulmonary resuscitation for out-of-hospital cardiac arrest make any difference to short term survival or long-term neurological outcomes.
Transendocardial Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells for Ischemic Cardiomyopathy (pg. 62): And for a final bit of dèja vu to start the new year, here is yet another trial showing that you can safely put transendocardial stem cells with autologous mesenchymal stem cells (MSCs) and bone marrow mononuclear cells (BMCs) into patients with ischaemic cardiomyopathy. What happens thereafter is anybody’s guess. Wake me up when there are some meaningful results.
BMJ 4 Jan 2014 Vol 348
Two Large Systematic Reviews of Coronary Stents: When I first started writing these reviews in 1998, coronary artery stents were quite a new thing: there were even radioactive stents on the market, but they didn’t last long when it was found that they caused arterial wall fibrosis. So how did they get on the market in the first place? Simply because regulatory control of medical device marketing was farcically lax then, and it hasn’t changed much since. I’ve recently heard a medical device manufacturer say quite openly that products simply can’t be subjected to lifetime safety testing because their business cycle simply couldn’t be run that way. New must always be pushed as better, until someone comes along and proves otherwise, at which point you take stock of the profits and move on.
This is not just the industry position: it also just about sums up the regulatory position for anything from absorbent dressings to left ventricular assist devices. This week’s BMJ has two large systematic reviews of coronary stents: one is a comprehensive network meta-analysis comparing durable polymer stents with biodegradable stents, and the other is “mixed treatment comparison meta-analysis” which also includes bare metal stents. Confused already? This is not entirely accidental: it is in the interests of device manufacturers to sow confusion as they go along. And since they pay for nearly all the trials, they can choose their case-mix and their comparators. What they will never tell you is what happens years down the line.
I genuinely admire these gigantic efforts: the first paper looks at data from 60 trials and the second looks at 126. What really puzzles me is that they both combine results from trials in acute coronary syndromes, for which there is a good evidence for PCI including stenting, and trials in stable coronary disease, where stenting is grossly overused and problems of case-mix become insuperable. Both articles plump for certain products as the safest, but I simply don’t believe that you can do that given the nature, duration, and quality of the data available.
Effect of β Blockers on Mortality after MI in adults with COPD: Atenolol was the wonder drug of the mid1980s, if your memory goes back that far. Even humble young GPs such as myself got word of ISIS-1 and duly prescribed it for all our post infarct patients, unless they have something called asthma. This was the era when there was much confusion between “asthma,” “chronic bronchitis,” and the new kid on the block, “chronic obstructive airways disease,” which shortly after morphed into COPD. Here is a study of British primary and secondary care databases which shows that only 39% of patients with COPD are prescribed a beta-blocker after MI in hospital, and that those who do receive one are twice as likely to be alive at three years. This is dire. Practice changed in the USA from 2001 onwards, after a paper in JACC by Krumholz and colleagues, and now 90% of COPD patients there receive beta-blockers after MI. It’s crazy that the message still hasn’t got through in Britain.
Role of Diuretics, β Blockers, and Statins in Increasing the Risk of Diabetes in Patients with Impaired Glucose Tolerance: Three classes of cardioprotective drugs have come under a cloud for inducing “diabetes:” β-blockers, thiazides, and statins. The NAVIGATOR study was a study of the effect of nateglinide and valsartan on 9306 subjects with impaired glucose tolerance, who were prescribed other drugs at the discretion of their doctors. So they form a high risk cohort for observing the effects of other medications as well as the effects of those they were randomised to receive as part of the trial. I am uneasy about the biases that creep in here, but let that be. “New diabetes” was a fasting glucose of 7mmol/L or above. Beta-blockers did not increase its occurrence but “diuretics” (presumably mostly thiazides) and statins did, by about a third. To discuss the relevance or otherwise of this would take too long here, so if you are interested I will point you to my recent exchange of views with a young American diabetologist on CardioExchange.