May 13th, 2014
A Second Darapladib Phase 3 Trial Misses Its Endpoint
Larry Husten, PHD
GSK said today that a large phase 3 trial of a once highly promising drug had failed to meet its primary endpoint. Last year the company announced that another phase 3 trial with the same drug had failed. GSK said it would “further analyse the data and better understand the findings” but that, for now at least, it would not seek regulatory approval for the drug.
The SOLID-TIMI 52 (Stabilisation Of pLaques usIng Darapladib – Thrombolysis In Myocardial Infarction 52) trial randomized more than 13,000 patients within 30 days of an acute coronary syndrome to receive either placebo or darapladib, an anti-inflammatory drug designed to stabilize atherosclerotic plaques. GSK reported today that the drug did not significantly reduce major coronary events. The full results of the trial will be presented at a future scientific meeting.
Last November GSK announced that the STABILITY trial (STabilisation of Atherosclerotic plaque By Initiation of darapLadIb TherapY) had failed to meet its primary endpoint. In that trial darapladib was compared with placebo in more than 15,000 patients with chronic coronary heart disease. The main results of the trial were then presented in March at the American College of Cardiology meeting and published simultaneously in the New England Journal of Medicine.
GSK said that the two trials had uncovered “no major safety concerns” with the drug. But an ongoing issue has been that darapladib causes unpleasant odors in feces and urine in some people taking the drug. Darapladib was originally developed by Human Genome Sciences. It was a key reason why GSK acquired that company.
May 13th, 2014
The Effect of Incarceration on the Epidemiology of Heart Disease
Emily Wang, MD, MAS
Emily Wang is an Assistant Professor at the Yale School of Medicine and Co-Founder of the Transitions Clinic Network, a consortium of 11 community health centers nationwide dedicated to caring for recently released prisoners and defining best practices for the health care of individuals leaving prison.
The United States incarcerates more people than any nation in the world, with currently 2.2 million individuals behind bars. Rates are the highest among African-American men, who have a one-in-three lifetime chance of being incarcerated. What does this have to do with cardiology? It turns out — more than you might think.
Aside from the fact that former prisoners have higher rates of hypertension, diabetes, and hospitalizations and deaths from cardiovascular disease (even after controlling for sociodemographic variables), the disproportionate incarceration of minority men may stymy the epidemiology of heart disease.
In 1978 the federal government restricted research on prison and jail inmates in medical studies, which was the result of decades of unethical research in correctional institutions. Currently, government regulations bar study subjects from participating once they are incarcerated, unless the study investigators apply for special permission through their institutional review board. If studies do not specify otherwise, community-recruited participants who enroll in studies cannot be followed while they are incarcerated. In certain jurisdictions, they cannot be followed after their release.
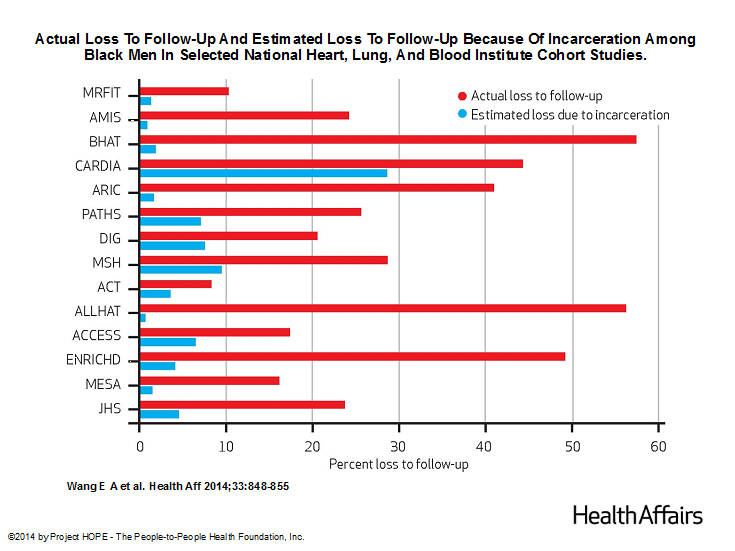
Because individuals who are already in ongoing studies must be dropped if they are incarcerated, this may compromise studies of health outcomes in minority populations, particularly studies involving black men, who are disproportionately incarcerated. In a study published in this month’s Health Affairs, we explored the effect of incarceration on follow-up rates of 14 prospective clinical studies funded by the National Heart, Lung, and Blood Institute (NHLBI). We chose to examine NHLBI cohort studies because it is the only institute that systematically requires the submission of all protocols and data for public use after trial completion.
We estimated that, during the past three decades, high rates of incarceration of black men have accounted for up to 65% of the loss to follow-up among black men in these studies.
The impact of incarceration was far less among white men, black women, and white women. These estimates suggest that the ability of those studies to examine racial disparities in health outcomes, as well as to understand the experience of this group, could be compromised. It becomes far more difficult for analysts to have access to a large number of cases so that they can draw statistically significant conclusions about cardiovascular disease, which is far more common among black men than white men, and is complicated by many factors that influence illness and death.
Under current circumstances, the results of longitudinal studies may fail to accurately represent the experience of black populations and may bias estimates of racial disparities by either excluding people in jail or prison or discontinuing longitudinal follow-up at the time of incarceration.
So what can be done? In 2006, the Institute of Medicine (IOM) convened a committee to explore “Ethical Consideration for Research Involving Prisoners.” The IOM suggested shifting from “a category based to a risk based approach to research review” [of protocols involving prisoners] and including a “framework” of “collaborative responsibility.” I think that this makes sense and that research subjects who consent in the community and then are incarcerated should be allowed to continue participating in observation research that poses minimal risk to research. But these changes have not been incorporated despite the IOM recommendation.
Perhaps the next step is to include patients in this conversation because of what is at stake: we know very little about the health risks of subjects during incarceration and that affects what we know about the health of black men in general.
May 13th, 2014
FDA Study Provides Some Reassurance About Boehringer Ingelheim’s Pradaxa
Larry Husten, PHD
In the latest development in its ongoing review of the new oral anticoagulant dabigatran (Pradaxa, Boehringer Ingelheim), the FDA today offered largely reassuring news about the sometimes controversial drug. The FDA study of over 134,000 Medicare patients found that dabigatran was associated with a reduced risk for ischemic stroke, bleeding in the brain, and death, compared with warfarin. But the study also found that dabigatran was associated with an increased risk for major gastrointestinal bleeding. There was no difference between the drugs in the risk for MI.
The FDA said the new study was “based on a much larger and older patient population than those used in FDA’s earlier review of post-market data, and employed a more sophisticated analytical method to capture and analyze the events of concern.” The findings were mostly consistent with earlier results from the pivotal RE-LY study, although an increased risk for MI found in the earlier study was not found in the new FDA study.
The FDA said that it will publish the Medicare study in the future and that it will continue to “to investigate the reasons for differences in major GI bleeding rates for Pradaxa and warfarin observed” in this study and in a previous study.
The FDA said it has not made any changes to the label for dabigatran and that it continues to believe that dabigatran has “a favorable benefit to risk profile.” The agency said that patients should not stop taking dabigatran or warfarin without first consulting with their physician.
Since its approval in 2010, dabigatran has been the subject of numerous safety questions and inquiries. According to IMS figures cited by the FDA, U.S. outpatient retail pharmacies have dispensed about 6.2 million prescriptions of dabigatran for 934,000 patients since 2010.
Here are the main findings of the FDA study:
Table 1. Incidence rates and adjusted hazard ratios comparing matched new user cohorts treated with Pradaxa 75 mg or 150 mg* or warfarin for non-valvular atrial fibrillation based on 2010-2012 Medicare data. Warfarin is the reference group.
|
Incidence rate |
Adjusted hazard ratio |
||
|
Pradaxa |
Warfarin |
||
| Ischemic stroke |
11.3 |
13.9 |
0.80 (0.67-0.96) |
| Intracranial hemorrhage |
3.3 |
9.6 |
0.34 (0.26-0.46) |
| Major GI bleeding |
34.2 |
26.5 |
1.28 (1.14-1.44) |
| Acute MI |
15.7 |
16.9 |
0.92 (0.78-1.08) |
| Mortality |
32.6 |
37.8 |
0.86 (0.77-0.96) |
* Primary findings for Pradaxa are based on analysis of both 75 and 150 mg together without stratification by dose.
May 12th, 2014
Selections from Richard Lehman’s Literature Review: May 12th
Richard Lehman, BM, BCh, MRCGP
CardioExchange is pleased to reprint this selection from Dr. Richard Lehman’s weekly journal review blog at BMJ.com. Selected summaries are relevant to our audience, but we encourage members to engage with the entire blog.
BMJ 10 May 2014 Vol 348
Influence of Healthy Candidate Bias in Assessing Clinical Effectiveness for ICDs: I was taught English in a northern British grammar school in the 1960s. This makes a me a bit curmudgeonly about much medical prose, especially from America. Consider this latest BMJ submission from Harvard: ” Lower risks of measured outcomes likely reflect unmeasured differences in comorbidity and frailty. The findings highlight potential pitfalls of observational comparative effectiveness research and support physician consideration of general health status in selecting patients for ICD therapy.” By spending a year in New England I’ve grown used to listening attentively to this kind of stuff and translating it as I go. “Likely” means “probably.” “Measured outcomes” means “recorded adverse outcomes.” The second sentence jumbles unrelated concepts. “Findings highlight potential pitfalls” is the sort of phrase that would have led to corporal punishment in my day. We were taught that writing was the organization of thoughts and words so that they followed a logical order and rhythm that would make them understandable. I can’t find any connection between the first and second parts of this sentence. If the findings show that important data about comorbidity and frailty are (likely) missing or unrecorded, how does that help physicians take them into account when selecting patients for ICD? And what is this “when selecting patients” about: shouldn’t that read “when discussing ICD implantation with patients?” This is paternalistic shared decision making at its traditional worst: the physician makes the decision on the basis of lousy evidence and the patient shares it.
May 12th, 2014
My Recent ABIM Maintenance of Certification Experience
John E Brush, MD
A petition to recall the American Board of Internal Medicine’s (ABIM) changes to Maintenance of Certification (MOC) has over 14,000 signatures. The petition calls for the ABIM to revert to the old method of simply certifying physicians using a test administered every ten years.
I didn’t sign the petition, but I am also unhappy. I think the ABIM ought to eliminate the every-ten-years secured exam and go with a more continuous testing approach. And I think the whole thing is way too expensive.
I am “grandfathered in” for Internal Medicine and General Cardiology, but not for Interventional Cardiology. I could probably coast to retirement, but I decided to maintain my certification. At the last ACC meeting, I got started.
First, I went to an MOC session headed by Rick Nishimura and Pat O’Gara on valvular heart disease. I have to say that it was the best learning experience I have had in many years. The case-based format was engaging and the content was skillfully delivered. And I received 10 points of Medical Knowledge MOC credit, as well as CME credit.
Next, I sat down with an ABIM staff member who was available at the ACC meeting to ask about the Performance Improvement Module. I told her that I have worked on quality improvement at my hospital for over 20 years. I attend a monthly committee meeting where we go over a mountain of data pertaining to the quality of cardiac surgery and catheterization laboratory procedures. I said that it didn’t make sense for me to design a little performance improvement project when I was already involved in quality improvement, big time. She agreed and said that, for me, the “Completed Project Performance Improvement Module (PIM)” was the way to go.
I came home and designed my own Completed Project PIM. I had a head start because my hospital participates in the National Cardiovascular Data Registry (NCDR) and I had already signed up for the NCDR Physician Dashboard. So I had ready access to my own NCDR data to use for my PIM.
I went to the ABIM website and found my way to the Completed Project PIM. The website led me to the Measures Library, but also gave me the option to submit my own measures for approval.
If you don’t use measures in the Measures Library, you are required to submit three of your own measures and these measures require ABIM approval. My measures were:
- Rate of radial access procedures among my patients undergoing diagnostic cardiac catheterization and percutaneous coronary intervention.
- Percentage of my patients undergoing percutaneous coronary intervention (PCI) procedures that were appropriate according to published appropriate use criteria.
- Percentage of my patients undergoing PCI who were discharged on a statin medication.
The NCDR dashboard gave me a ready data source for these measures. Others may want to design different measures, built around the data that are available on the NCDR dashboard, or perhaps the PINNACLE registry. The ABIM requires measurement from two time periods and a minimum of 25 patients.
On the ABIM website, I had to fill in the title and description of each measure, and a reference to a guideline recommendation that justified each measure. I filled in the form and submitted my measures by hitting the submit button on the website. In less than 24 hours, I received an email from the ABIM approving them.
The website automatically knew that my measures were approved and it led me to the next step of reporting my results. It asked me a few additional questions, and that was it! All told, over 2 days I probably put about 3 hours into the activity. With the Completed Project PIM, the ABIM acknowledged that I had already done the work. It was a matter of documenting what I have been doing already.
Let’s face it. The public will always demand that doctors are certified by an independent, objective certifying organization. None of us likes the added work that certification creates, but it is unavoidable. The changes in the ABIM requirements have created a lot of confusion, but the website helps you navigate through the changes, and for me, the ABIM staff was available and helpful.
I would rather have a series of small projects and learning modules, creating an atmosphere of continuous learning and improvement, rather than the big ten-year exam. To me, the petition asked for the wrong thing. I would eliminate the big exam and stick with the other stuff, which isn’t so bad after all.
May 9th, 2014
Routine Defibrillation Testing Not Required During ICD Implantation
Larry Husten, PHD
Although commonly performed, routine defibrillation testing has never been shown to be safe or necessary. Now a new study, presented yesterday at the Heart Rhythm Society meeting in San Francisco, offers evidence that although routine testing is generally safe it may increase complications without producing any improvement in outcomes.
In the SIMPLE (Shockless Implant Evaluation) trial, sponsored by Boston Scientific and led by researchers at McMaster University in Hamilton, Ontario, 2,500 patients in 18 countries undergoing ICD implantation were randomized to routine defibrillation testing (DT) or no DT.
After three years of followup there was no significant difference in the primary endpoint of the trial, which was the rate of death due to arrhythmia or the failure of a first shock to terminate a lethal arrhythmia (7.2% in the no-DT arm versus 8.3% in the DT arm, hazard ratio 0.86; CI 0.65-1.14, non-inferiority p<0.001). There was no difference between the groups in total mortality.
The authors concluded that defibrillation testing “does not improve ICD shock efficacy or reduce mortality.” Although routine testing is “generally low risk,” complications may occasionally take place. Because of these facts, “the ‘no testing’ approach to routine ICD implantation should be preferred.”
“This is the first time the relationship between defibrillator testing and patient outcome has been studied independently in a randomized setting and with these results, we can confidently say that defibrillator testing, although safe, is not necessary at the time of ICD implantation,” said the lead author of the study, Jeff Healey, in a press release. “These results should change physician practice and could help reduce costs without compromising patient outcomes.”
May 8th, 2014
Novel Antiplatelet Agent Vorapaxar Gains FDA Approval
Larry Husten, PHD
The FDA today approved vorapaxar (Zontivity), Merck’s once-troubled platelet receptor antagonist, to reduce the risk of myocardial infarction (MI), stroke, cardiovascular death, and revascularization procedures. The drug is approved for use in people with a history of MI or peripheral arterial disease. The approval represents an amazing turnaround for a drug that has experienced nearly as many ups and downs as an amusement park roller coaster.
Vorapaxar is the first in a new drug class known as protease-activated receptor-1 (PAR-1) antagonists. By preventing the ability of platelets to stick together and form blood clots, the drug can help prevent MI and stroke. But it also increases the risk of bleeding, including life-threatening and fatal bleeding. Because the risk of serious intracranial bleeding is especially high in people who have had a stroke, a transient ischemic attack, or bleeding in the head, use of the drug is contraindicated in these groups. The drug’s label includes a boxed warning about the risk of bleeding. The FDA said the drug will be dispensed with a patient Medication Guide.
Vorapaxar appeared to be all but dead a few years ago after unacceptably high serious bleeding rates were found in two large clinical trials. But hopes for the drug resurfaced with a new analysis of one of those trials, the TRA2P trial. In a statement to the press, Ellis Unger, director of the FDA’s Office of Drug Evaluation I, said that the trial showed that “in patients who have had a heart attack or who have peripheral arterial disease, this drug will lower the risk of heart attack, stroke, and cardiovascular death… Zontivity lowered this risk from 9.5 percent to 7.9 percent over a 3-year period – about 0.5 percent per year.”
In January the FDA’s Cardiovascular and Renal Drugs Advisory Committee voted 10-1 in favor of approval for vorapaxar.
May 8th, 2014
Orbital Atherectomy Revisited
Kush Agrawal, MD
My attendance of the Cardiovascular Research Technologies (CRT) symposium — held in Washington, D.C. in February — was an exciting experience and a conference that I would highly recommend. Broad topics were channeled into streams of focus: complex PCI, lesion prep, peripheral intervention, and emerging applications towards endovascular approaches for critical limb ischemia.
While attending, I was able to observe and participate in a live case demonstration of orbital atherectomy, currently available in the form of the Diamondback Orbital Atherectomy System (OAS) manufactured by Cardiovascular Systems, Inc.
Where does OAS stand? Post-approval experience is limited, as the device was approved by the FDA last October, with individual operators endorsing an ease of use with the device, an enhanced fidelity of 1:1 burr to console feedback vs. traditional rotational atherectomy (RA), shorter fluoroscopy times, and lower contrast volume use.
For the observer, CRT’s unique approach to the live case demonstration of complex cases afforded an enhanced educational experience that should be modeled in future conferences.
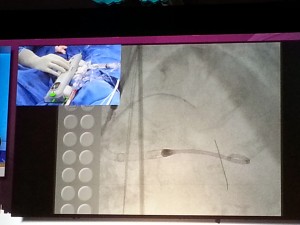
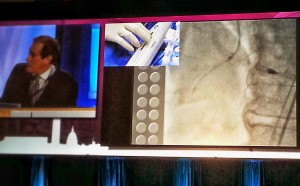
Dr. Jeffrey Moses from Columbia University led an expert panel (located on the right in the photograph below).
The live demonstration — on two large high definition (HD) projection screens — was performed by Drs. Samin Sharma and Annapurna Kini and colleagues at the Mt. Sinai cath lab (on the right screen in the photograph below), with preparatory educational slides (on the screen to the left), consistent with the pre-existing live case format available to the community on the Mt. Sinai website (e.g., coronary cases and peripheral cases).
The third screen on the far left (not pictured) contained a real-time Twitter-format feed for audience Q&A and participation. This was accomplished by co-opting the use of individual smartphones and tablets, seamlessly integrated through the CRT conference website.
This setup enabled the demonstration to maintain a focus that was continually redirected by the audience questions and afforded us a chance to provide answers to questions outside of the expert panel in real time.
The senior Mt. Sinai operators presented a complex case of a septuagenarian with recurrent angina who refused CABG. The patient had an LVEF of 30% and 3-vessel obstructive CAD, a highly calcified 80% proximal to mid LAD heterogeneous lesion, a patent mid left circumflex stent, and an ostial dominant 40% RCA with 50% at the mid-vessel. With the patient under monitored anesthetic care, the case initiated with bilateral femoral access with a 7F sheath on the right and a 9F sheath on the left and an Impella CP® (Abiomed) was inserted.
Lesion prep of both the LAD and RCA (no prior FFR or viability data were available at time of presentation) with the OAS appeared technically simple and straightforward in the hands of the skilled operators.
OAS currently costs about twice as much as traditional RA. Do you see it eventually supplanting traditional RA or as simply a “new toy” with a niche application?
May 8th, 2014
Selections from Richard Lehman’s Literature Review: May 8th
Richard Lehman, BM, BCh, MRCGP
CardioExchange is pleased to reprint this selection from Dr. Richard Lehman’s weekly journal review blog at BMJ.com. Selected summaries are relevant to our audience, but we encourage members to engage with the entire blog.
NEJM 1 May 2014 Vol 370
Darapladib for Preventing Ischemic Events in Stable Coronary Heart Disease (pg. 1702): Britons, mourn. Our biggest drug company, GlaxoSmithKline, had a potential blockbuster on its hands. Darapladib would stabilise unstable plaque, everybody would want to take it, and GSK would make billions. But, although darapladib is a selective oral inhibitor of lipoprotein associated phospholipase A2, which is an enzyme associated with plaque instability, 8000 people with stable coronary artery disease took it for three years with outcomes no different from the 8000 people who took placebo. Britons rejoice. The NHS will not have to pay for millions of prescriptions for darapladib. We have enough wonderfully effective substances already for the secondary prevention of cardiovascular events: fruit, fish, alcohol, aspirin and statins. And nuts.
BMJ 3 May 2014 Vol 348
Discrepancies in Autologous Bone Marrow Stem Cell Trials and Enhancement of EF (DAMASCENE): A while back I suggested in these reviews that the major journals should refuse any further papers about stem cells for regenerating myocardium until one of them showed some positive effects on patient important outcomes. Ten years ago, this concept was exciting and it was reasonable for hope to triumph over cold reality; but, as the reality has stayed cold, the converse now applies. Yet, as a recent Cochrane review update suggested, there are some studies that have faintly positive outcomes, though with a high risk of bias. Darrel Francis and the DAMASCENE team went further and performed the exacting task of looking at all the trials to discover whether their sums add up. The results of this brilliant analysis are quite damning: there was a direct relationship between figures that contradicted each other and alleged increases in ejection fraction following autologous bone marrow cell administration.
Dietary Fiber Intake and Mortality Among Survivors of MI: People who go out of their way to eat cereal fibre (The BMJ actually spells it “fiber” here) are not typical. They are “health conscious” and are likely to wear sandals, ride bicycles, grow their own vegetables, and all sorts of other things that I might do myself were I ever to acquire the skill and inclination. They have a low risk of myocardial infarction and a higher chance of surviving it if they do experience an event. This is not surprising. What is surprising is that anyone supposes you can isolate this component of behavior (sic) while adjusting for all the rest.
Book of the Week: The Science of the Art of Medicine by John Brush
Like all of you, I rarely read a medical book at all, let alone from cover to cover. It’s become a truism that all medical texts are out of date before they are printed. This book is a rare exception, and I would even go so far as to say that it will be read in a hundred years’ time and still have valuable things to say. Moreover it does not have covers and it is not printed.
You were among the brightest of your class at school. When you got a place at medical school, you realised that you only had to persevere for five or six years, well within the limits of your abilities, and you would have a job for life. You were taught innumerable facts and skills, and then you were cast into the real world of hospital medicine. You were often tired, you sometimes wished for patients to die so that you could get some sleep, you made mistakes and they did die, and you lay awake and wondered what you should have done differently. Gradually you learnt your own coping skills: things first done arduously became instinctive, and you also learned through the camaraderie of those who shared your stresses, mistakes, and triumphs.
That is how you learned the “Art of Medicine.” Do you feel it was the right way? Do you feel you now know how to do it better than anyone else? If so, there is absolutely no need to read John Brush’s book. You should instead write one of your own. But I bet it won’t be as good.
Here is a fuller account of what I think about it. It is free, it is beautiful, and it is short. I defy any clinician to read it and not benefit.
May 6th, 2014
FDA Approves New Omega-3 Supplement
Larry Husten, PHD
The FDA has approved a new omega-3 supplement for the treatment of adults with severe hypertriglyceridemia, defined as triglyceride levels 500 mg/dL or higher. The drug, which will be marketed under the brand name of Epanova, is manufactured by AstraZeneca, which acquired the drug when it purchased Omthera Pharmaceuticals in 2013.
AstraZeneca said that Epanova is the first omega-3 formulation approved by the FDA in free fatty acid form. Epanova will be available in 1-g capsules and has been approved for 2-g and 4-g dosages, which can be taken with or without food.
There are currently two other prescription formulations of omega-3 supplements: Lovaza from GSK and Vascepa from Amarin Pharmaceuticals. All three are approved only for the treatment of severe hypertriglyceridemia. Last year the FDA turned down a request from Amarin to allow an expanded use of Vascepa in people with more common forms of dyslipidemia. The expanded indication for Vascepa is now being studied in the ongoing REDUCE-IT trial, which is scheduled to enroll 8000 patients.
The use of Epanova in an expanded population will be studied in the 12,000-patient STRENGTH study, headed by the Cleveland Clinic’s Steve Nissen, Michael Lincoff, and Stephen Nicholls.