October 13th, 2010
Transfusions and Cardiac Surgery: “A Major Concern”
Larry Husten, PHD
One new study in JAMA demonstrates very wide differences among hospitals in the use of transfusions during cardiac surgery. A second study finds no differences in outcome based on transfusions. Two editorialists write that “continued inappropriate transfusions among hospitals is a major concern.”
Bennett-Guerro and colleagues analyzed the Society of Thoracic Surgeons Adult Cardiac Surgery Database to assess the rate of perioperative blood transfusions in over 100,000 patients who underwent CABG in 2008. The transfusion rate across CABG sites ranged from 7.8% to 92.8% for red blood cells (RBCs) and from 0% to 97.5% for fresh-frozen plasma. Although geographic location, academic status, and hospital volume were all significant factors, these three characteristics accounted for only a small (11.1%) portion of the variation in risk-adjusted RBC usage. The investigators write that “to our knowledge, there has never been a large randomized trial of the safety and efficacy of blood transfusion in cardiac surgery; therefore, some of the variability we observed may be due to honest differences between clinicians in the perceived benefits and risks of transfusion.”
In an attempt to shed some light on the topic, Hajjar and colleagues performed the propspective Transfusion Requirements After Cardiac Surgery (TRACS) clinical trial. They randomized 502 patients at a single university hospital in Brazil to either a liberal strategy of blood transfusion or a restrictive strategy (hematocrit goal of > 30% or >24%). Some 78% of patients in the liberal strategy group received a transfusion, compared with 47% in the restrictive-strategy group. The composite endpoint of 30-day mortality, cardiogenic shock, acute respiratory distress syndrome, or acute injury requiring dialysis or hemofiltration did not differ significantly between the two groups (10% vs. 11%, P=0.85).
In an accompanying editorial, Aryeh Shander and Lawrence Goodnough observe that Society of Thoracic Surgeons ratings of cardiac surgery programs do not include RBC transfusions as a quality indicator and suggest that “it may be time for patient blood management to gain status as a performance indicator by accreditation agencies such as the Joint Commission or as a quality indicator by professional organizations.” They conclude:
“When evaluating a hemoglobin level, treating physicians must resist the temptation to ‘first do something’ and temper this temptation with a philosophy of ‘first do no harm’ to achieve the optimal balance of providing the best risk-benefit and cost-effective outcomes of transfusion therapy for patients.”
October 12th, 2010
Heart Failure and Resource Use at the End of the Road
Larry Husten, PHD
Two studies of heart failure populations — one conducted in the U.S. and one in Canada — shed light on patterns of resource use in the last 6 months of life. Both studies appear in the Archives of Internal Medicine.
Kathleen Unroe and colleagues retrospectively analyzed resource use in a cohort of nearly 230,000 U.S. Medicare beneficiaries with heart failure who died between January 1, 2000 and December 31, 2007. Although patient use of hospice services increased over the course of the study, overall use of resources and costs also increased.
Padma Kaul and colleagues analyzed resource usage in some 33,000 elderly patients with heart failure who died between January 1, 2000 and December 31, 2006 in Alberta, Canada. Although costs for the patients continued to increase during the study, the number of hospitalizations and in-hospital deaths decreased and the use of outpatients services increased.
In an accompanying editorial, Rosemary Gibson notes that the two studies were unable to answer whether the patients received appropriate care. She writes that “conversations that allow the patient to describe what is important as he or she lives life with serious illness or near life’s end should be paramount in guiding the course of treatment.”
October 11th, 2010
Obesity: Good News and Bad News
Larry Husten, PHD
Two new trials and accompanying editorials published online in JAMA offer hope that lifestyle interventions can result in significant weight loss. The bad news: The results are fairly modest, and it is difficult to obtain reimbursement for lifestyle interventions.
In one study, Cheryl Rock and colleagues compared usual care with a program that included free prepared meals and counseling in 442 overweight or obese women. After 2 years, weight loss in subjects who received prepared meals and either center-based or telephone-based counseling was significantly greater than weight loss in subjects who received usual care (7.4 kg and 6.2 kg vs. 2 kg, P <0.001 for intervention effect). But in an accompanying editorial, Rena Wing writes that the results “probably represent a best-case scenario” and that “it is time to directly compare the outcomes achieved in a variety of different commercial weight loss programs and to examine whether providing these programs free of charge to participants would be a cost-effective approach.”
In a second study, Bret Goodpaster and colleagues randomized 130 severely obese adults (mostly women) to diet and exercise for 12 months, or to a 6-month period of diet alone followed by 6 months of diet and exercise. At 6 months, people in the combination diet-exercise group lost more weight than the diet-only group, but at 12 months, weight loss was similar in the two groups. In an accompanying editorial, Donna Ryan and Robert Kushner note that reimbursement for nonsurgical treatment of obesity is rare, adding: “Physicians should not be discouraged from implementing nonsurgical medical care approaches in this population, but payers need to rethink their policies.”
October 8th, 2010
Abbott Withdraws Sibutramine from U.S. Market
Larry Husten, PHD
Abbott has pulled its weight-loss drug sibutramine (Meridia) from the U.S. market. A safety communication from the FDA said the “drug may pose unnecessary cardiovascular risks to patients.” Earlier this year the drug was withdrawn in Europe.
Sibutramine was approved by the FDA in 1997. The FDA recommendation is based upon a recent analysis of data from the Sibutramine Cardiovascular Outcomes (SCOUT) trial, in which patients taking sibutramine had a 16% increase in risk for cardiovascular events.
October 8th, 2010
Analysis of ADVANCE Explores Role of Hypoglycemia
Larry Husten, PHD
A new analysis of the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation) trial sheds light on the role of hypoglycemia in recent trials of glucose control. In a paper in the New England Journal of Medicine, the ADVANCE investigators report that 2.1% of 11,140 patients with type 2 diabetes developed severe hypoglycemia in the course of the trial. Patients with severe hypoglycemia had a significantly elevated risk of major macrovascular events, major microvascular events, and death from a cardiovascular cause or from any cause.
However, the investigators did not find either a temporal or a dose-response relationship between hypoglycemia and outcome. The authors conclude that “although our findings cannot exclude the possibility that severe hypoglycemia has a direct causal link with these outcomes, they suggest that it is as likely to be a marker of vulnerability to a wide range of adverse clinical outcomes.”
October 7th, 2010
Rolofylline Fails in Heart-Failure Trial
Larry Husten, PHD
In a report in the New England Journal of Medicine, Massie and colleagues randomized 2033 patients hospitalized with acute heart failure and impaired renal function to receive intravenous roloflylline or placebo. Earlier studies had suggested that the use of an adenosine A1-receptor antagonist might be beneficial in this patient population.
There was no difference between the two groups in the primary endpoint, which was based on survival, heart-failure status, and changes in renal function. At 60 days the rate of death or readmission for cardiovascular or renal causes was similar in both groups. The authors concluded that rolofylline “does not show promise in the treatment of acute heart failure with renal dysfunction.”
October 7th, 2010
Genome Studies Pool Data to Gain Power
Larry Husten, PHD
Leaders of several genome-wide association studies have agreed to collaborate and combine their data in the hope that by dramatically raising the sample size of their studies, they will “contribute to our understanding of the role of common genetic variation on risk for CAD and MI.” In an article in Circulation: Cardiovascular Genetics, Michael Preuss and colleagues report the formation of the CARDIoGRAM (Coronary ARtery DIsease Genome-wide Replication And Meta-analysis) consortium.
“Only a small proportion of the inheritability of CAD has been explained,” said Heribert Schunkert, a spokesperson for the consortium, in an AHA press release. “By pooling all of the published and unpublished data, we hope to make discoveries that might have been overlooked. Given that up to 2.5 million comparisons are carried out, in parallel, for each whole-genome scan, distinguishing between true and false associations has been difficult.”
October 7th, 2010
Case Closed: What’s at the Heart of this Patient’s Problem?
CardioExchange Editors, Staff
We’ve wrapped up part II of our latest case: A 53-year-old smoker with a history of diverticulitis and prior GI bleeding presents with lightheadedness and bright red blood per rectum. James Fang tells us his recommendations, and Anju Nohria tells us how the patient actually fared. Are you surprised by the outcome? Share your thoughts here.
October 6th, 2010
Study Finds No Evidence for Clopidogrel-Omeprazole Interaction
Sanjay Kaul, MD and Larry Husten, PHD
A large clinical trial has found no evidence that omeprazole interferes with the cardiovascular efficacy of clopidogrel. COGENT (Clopidogrel and the Optimization of Gastrointestinal Events Trial) randomized 3873 patients eligible for dual antiplatelet therapy to receive aspirin, clopidogrel, and either omeprazole or placebo. The COGENT investigators had planned to enroll 5000 patients, but the trial was terminated early when the sponsor ran out of money.
Gastrointestinal events occurred in 2.9% of the placebo group compared with 1.1% of the omeprazole group (p<0.001). There was no significant difference in the rate of cardiovascular events between the two groups (5.7% in the placebo group versus 4.9% in the omeprazole group; p=0.096). In their report in the New England Journal of Medicine, the authors write that the study “provides reassurance that there is no clinically significant cardiovascular interaction between PPIs and clopidogrel.”
Sanjay Kaul provided the following points on the clinical implications of COGENT:
- No clinically relevant adverse CV interaction between clopidogrel and omeprazole in COGENT, a prospective randomized trial. The cardiovascular results refute the previous findings based on observational studies; however, they confirm the post hoc assessments of other randomized trials such as CREDO and TRITON. The current findings do not support the need to avoid concomitant use of omeprazole in patients treated with clopidogrel. However, given the limited number of cardiovascular events in the COGENT trial and the fact that the study was conducted predominantly in Caucasians where the frequency of loss-of-function CYP2C19 genetic polymorphism is low (2-3%), one cannot completely rule out the possibility of an adverse interaction between clopidogrel and omeprazole (or other PPIs) in other populations where the frequency of CYP2C19 genetic polymorphism is high (for example, Asians).
- Substituting an H2 receptor antagonist such as ranitidine for a PPI, especially in those for whom there is no compelling indication for a PPI, should remain an important consideration during concomitant therapy with clopidogrel.
- Interpret information from observational studies with caution, as they are often subject to confounding and bias and can yield potentially misleading results.
- The findings raise questions about the relationship between ex vivo platelet function assays and clinical outcomes. Platelet function assay-guided treatment decisions are not ready for prime time.
- Finally, should regulatory agencies and professional societies change label or practice recommendations based on limited data?
October 6th, 2010
The Skinny on Drug-Eluting Stents (DES)
Richard A. Lange, MD, MBA
(“All we want are the facts, ma’am.” –Joe Friday, Dragnet)
Having trouble keeping up with DES and the recent stent studies? Want a brief tutorial? To learn the top six things every cardiologist should know about DES, read on…
1. What they do. DES are superior to both bare-metal stents and angioplasty in reducing the incidence of clinical restenosis and the need for reintervention after PCI. Clinical restenosis occurs in 30% to 50% of patients after angioplasty, in 20% to 30% after bare-metal stent implantation, and in about 10% after DES implantation.
Compared with bare-metal stents, DES confer an increased risk for stent thrombosis months (and years) after placement. Therefore, patients who receive DES are placed on dual antiplatelet therapy for at least a year, which increases their risk for a bleeding complication.
2. What they don’t do. DES do not reduce the risk for death or MI in patients with stable ischemic heart disease. This is a commonly held misperception (among patients and physicians). Sorry to burst this bubble.
3. First-generation stents. Three first-generation DES are available in the U.S.: the sirolimus-eluting CYPHER stent (Cordis), the paclitaxel-eluting TAXUS stent (Boston Scientific) and the zotarolimus-eluting ENDEAVOR stent (Medtronic). Outcomes after sirolimus- and paclitaxel-eluting stent implantation appear to be similar; however, the zotarolimus-eluting stent appears to be less effective (higher all cause-mortality, MI, and target-lesion restenosis [TLR] at 18 months than the sirolimus-eluting stent in the SORT OUT III study). Hard to justify using the zotarolimus-eluting stent.
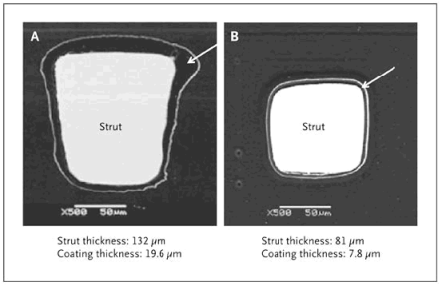
4. Second-generation stents. Second-generation DES are designed to improve stent deployment, safety, and efficacy by altering the composition and thickness of the metal struts, the copolymer, and/or the antiproliferative drug. Second-generation DES release everolimus, a semisynthetic sirolimus analogue, from a thin coating of a biocompatible fluoropolymer on a flexible cobalt–chromium stent frame with thin struts.
Figure 1. Scanning Electron Micrographs of a Paclitaxel-Eluting Stent and an Everolimus-Eluting Stent. Shown are scanning electron micrographs of a cross-section of a paclitaxel-eluting stent strut (TAXUS Express, Boston Scientific) (Panel A) and an everolimus-eluting stent strut (XIENCE V, Abbott) (Panel B). As compared with the everolimus-eluting stent, the paclitaxel-eluting stent has a thicker strut and a thicker polymer coating (arrow). Reprinted with permission from Doostzadeh et al. Recent progress in percutaneous coronary intervention: Evolution of the drug-eluting stents, focus on the XIENCE V drug-eluting stent. Coron Artery Dis 2010; 21:46-56.
____________________________________________________________
Two everolimus-eluting stents are currently available in the U.S.: the Xience V (Abbott) and the Promus (Boston-Scientific “private label”). Don’t be fooled. The Promus stent is actually the Xience V stent from Abbott (i.e., like bottling the same wine under 2 different labels…vive la différence!)
Why sell 2 second-generation stents that are identical? DES is big business, with a world market of approximately $5 billion annually and over 2 million stents implanted already. Not surprisingly, therefore, both companies want a share of the market.
The second-generation stents cost about $300 more than the first-generation stents.
5. Stent Wars: Who’s Winning? In two studies (COMPARE and SPIRIT IV) with 2-year follow-up, the second-generation everolimus-eluting stent was superior to the first generation paclitaxel-eluting stent, with fewer stent “failures” (i.e., composite cardiac death, MI, TLR and stent thrombosis).
In two studies (SORT OUT IV and ISAR 4) with 9 and 24 months of follow-up, the second-generation everolimus-eluting stent was noninferior to the first-generation sirolimus-eluting stent, with a trend towards less restenosis with the everolimus-eluting device after longer follow-up.
6. The Bottom Line. Studies have shown that the everolimus-eluting stent is comparable to the less-expensive sirolimus-eluting stent (although longer follow-up and additional studies are needed to confirm this) and superior to the paclitaxel-eluting stent.
In patients with diabetes, who comprise 20% to 30% of patients undergoing PCI, the second-generation stents do not appear to be more effective than the first-generation (less expensive) stents.