April 11th, 2012
Anticoagulation Conundrum
Tariq Ahmad, MD, MPH and James Fang, MD
A 75-year-old woman presents to a general cardiology clinic for the first time. She has a history of atrial fibrillation, sick-sinus syndrome requiring a permanent pacemaker, hypertension, and dyslipidemia. She has no specific complaints other than shortness of breath on exertion. Her medications include aspirin (81 mg daily), carvedilol (25 mg twice daily), lisinopril (5 mg daily), and furosemide (40 mg daily).
The woman has just moved in with her daughter, who lives in the area. She helps take care of her grandchildren and buys the groceries for the entire family.
A transthoracic echocardiogram reveals severe left-ventricular hypertrophy and an LV ejection fraction of 45% (atrial fibrillation is consistently rate-controlled). The patient has mild aortic, mitral, and tricuspid regurgitation. A mass detected in the left atrium does not opacify with contrast, suggesting the possibility of a thrombus.
The patient has brought medical notes from previous physician visits, showing that she was hospitalized 3 years ago with a severe headache. She was found to have a subarachnoid hemorrhage that required embolization. At that time, she had an INR in the 2.0 to 2.4 range on warfarin, which was discontinued in light of the subarachnoid hemorrhage.
A year later, the patient was hospitalized with severe bilateral calf pain. A CT scan revealed thrombotic occlusion of the right common iliac and right external iliac arteries. She underwent bilateral iliac and left profunda thrombectomy, with stent placement in the right common iliac artery. After much discussion between the cardiologists and neurosurgeons, the decision was made to re-initiate anticoagulation with warfarin.
Within 6 months after discharge, the patient was readmitted with a massive GI bleed requiring 8 units of packed red blood cells. At the time of this admission, her INR was 2.2. Subsequently, all anticoagulation was stopped. Six months before the patient’s current clinic visit, she had re-initiated aspirin therapy, which she tolerated well.
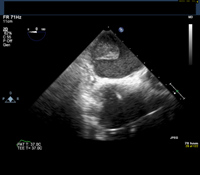
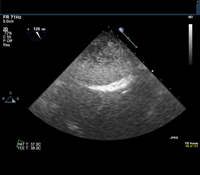
Given this history, the patient is scheduled for an elective transesophageal echocardiogram the day after presenting to the clinic. The images below show the TEE findings: a large 5.5-cm x 2.7-cm heterogeneous multilobulated mass, attached along the posterior wall of the left atrium, which likely represents a thrombus. (Click to enlarge the images.)
Questions:
1. Would you anticoagulate this patient? If so, which agent would you use?
2. Would you perform any further diagnostic testing?
Response:
April 24, 2012
This type of scenario, in which the risks for bleeding and clotting are roughly balanced, is common in clinical practice. The notion that “all bleeding stops” favors anticoagulation (given that stroke is irreversible), but the ultimate extension of that course of action is fatal hemorrhage. This patient has had several life-threatening episodes of both hemorrhage and thromboembolism, so applying traditional risk modeling is not likely to be appropriate.
Discussion of agents other than warfarin in this setting would be largely academic, given that this patient would not be included in a clinical trial.
Unfortunately, an evidence base to inform us here is scant, so we must rely on good old-fashioned common sense, keeping in mind the adage to do no harm. A reasonable empiric approach is an unfractionated heparin “stress test.” Acute bleeding would answer the immediate question of whether to initiate anticoagulation, although this method would be limited in its ability to predict future bleeding. If the UFH is tolerated, I would consider a transition to oral warfarin with an INR target of 2.0 to 2.5. One unifying diagnosis would be amyloidosis, senile or primary.
Finally, I, too, would have tried to get more imaging of the posterior left-atrial mass. However, I have seen this sort of finding after surgical or percutaneous ablations for atrial fibrillation.
Follow-Up:
April 30, 2012
Thank you all for your comments — I wish we had consulted fellow cardiologists in real time. The patient was started on a heparin drip, which she tolerated without any evidence of bleeding. Consistent with a finding of intra-atrial thrombus, she underwent a cardiac CT scan (MRI was not done because of her pacemaker). A diagnosis of amyloidosis was considered and ruled out. The patient was transitioned to warfarin and thus far has done well without any further evidence of bleeding or thromboembolism.

First of all, because she is 75 years old, i should know her renal function, cognitive level and the functional status to decide if I could prescribe her dabigatran at dose of 110 mg every 12 hours.
I would not restart warfarin.I wonder if the L atrial mass should be evauated further.
MRI to assess for possible tumor and therefore plan resection.
This is a great case, Tariq. I also feel the mass needs to be defined more- as it does not seem to be in the left atrial appendage based on these images, which is a little unusual. Thus, perhaps a CT scan might be useful to characterize further (MRI is not an option due to PPM). If the mass is confirmed to be thrombus, I would anticoagulate this lady with warfarin again because of her risk of stroke. I think dabigatran would be difficult in her because of her bleeding tendencies even when her INR is in the therapeutic range. Rivaroxaban does seem to be safer in patients >75 (at least based on EINSTEIN PE data), but her pre-disposition to bleeding events (on top of clotting events) again makes me cautious against using this. It is strange to me that she has such a mixed bleeding and clotting diathesis, but I cannot think of a unifying diagnosis, except perhaps GI cancer or chronic DIC.
I would initiate IV heparin for a few days, probably without boluses, and if she tolerates it, then switch to warfarin. I would avoid newer agents because if she bleeds it would be harder to stop it. In addition, it may help to know where she was bleeding from at the time of her GI bleeding. I also agree that she should undergo hematology evaluation.
This is certainly a difficult case in that there is no easy answer as any pathway carries significant risks. Dabigatran is only approved at 75mg or 150mg in the US. The 150mg dose was associated with increased risk of GI bleeding compared to warfarin in te RE-LY study, so would likely not be appropriate in this patient with a history of recent GI bleeding. As far as I’m aware, there is no published evidence to support the 75mg dosage of dabigatran in the non-valvular A-fib, despite the FDA approval of it. More information needs to be known about the etiology of the GI bleed such as PUD/UGIB vs LGIB as treatment treatment for H. pylori or PPI therapy for an ASA induced ulcer could reduce the risk of recurrent up GI bleeding. Rivaroxaban was non-inferior to warfarin in ROCKET AF for the primary end-point and had equal rates of bleeding.
I assume from above information that the right iliac arterial occlusion was thought to be from a systemic embolism from the atrial fibrillation as opposed to primary arterial thombus formation secondary to PAD +/- an acquired thrombophilia.
Given two bleeding events (both potentially life threatening) vs one thomboembolic event (potentially limb threatening), I think the risk of anticoagulation outweighs the benefit, though I think it depends on which the patient is more concerned about.
I do think it would be reasonable for the patient to undergo further evaluation for the etiology of the left atrial mass/thrombus, though I’m not sure how much it would change my management.
While there isn’t much evidence to support this, perhaps putting the patient on aminocaproic acid may help reduce the risk of bleeding if anticoagulation is tried again.
Anticoagulation is indicated and apiximab would probably be the drug of choice, since causes less bleeding compared to warfarin.
To reduce the risk of further GI bleeding a PPI should also be initiated if erosion or ulcer were the source of bleeding. If not, further GE exams should be performed.
Echocardiographic findings need to be clarified, in particular severe LVH in old women without significant aortic valve stenosis. This is suggestive of restrictive cardiomyopathy and given the clinical picture my tip would be hypereosionophylic syndrome so I would recommend diagnostic workout in that sense, including Churgg-Strauss syndrome.
Can you clarify whether you mean abciximab or apixaban? (It just occurred to me that this confusion of names might become a clinical issue in the future.)
Sorry, I ment apixaban, you’re right about name issue.
Excellent comments thus far! As John Ryan pointed out, this was indeed an unusual place for an atrial thrombus. While a cMRI might have been our first choice for characterization of this mass, we were unable to do this due to her PPM. We did get a cardiac CT that confirmed that this was most likely a thrombus and not a cardiac tumor. She was seen in consultation by our hematology colleagues, who were unable to unearth an inborn or acquired predisposition to thromobosis or bleeding. We got records from her previous hospitalization for GI bleeding and it appeared that this had had a diverticular source. As Dr. Harrell pointed out, the peripheral arterial occlusion was thought to be from AF induced systemic embolisation.
Will have to check, but I’m almost certain that the novel anticoagulant trials probably systematically excluded those with a prior sub-arachnoid bleed. One approach might be an in-hospital trial of unfractionated heparin for a few days and a revaluation of the mass would give a good sense of whether this is a thrombus. If the mass shrinks, one might consider warfarin or subcutaneous heparin and consider relook to be able to stop it when the “thrombus” dissolves. If turns out to be a tumor and need for surgery arises, would also exclude LAA. For the dyspnea- There maybe need for diuretic dose titration; there is also enough room on the lisinopril dose.
Also-would like to hear more from the MD who wanted to try abciximab.
As I wrote above, I ment apixaban, since this patient perfectly fits in ARISTOTLE study design. The bleeding risk there was significantly lower than with warfarine (by 31%)
thanks for this complicated case,which need careful approach
she had 2 episodes of life threatening bleed as side effect of anti-coagulation even inr were in-therapeutic range,and when stopped she got arterial thrombus which need thromboectomy,
i,think ,first we have to remove this atrial thrombus surgically,and meantime to do thrombophilia lab assessment.then to statrt lmwh. and later-on warfarinze the patient.
thanks again
waiting your-replay.
A Common case scenario in day-to-day practice. Patient is 75Y and has had multiple episodes of thromosis; she need further evaluation for secondary causes for thromboembolism; mainly to rule out underlying malignancy. I think it is very important to do gastroscopy and colonoscopy prior to antigoagulation.
Finally, she should be given the benefit of anticoagulation, as she is high risk patient to have thromboembolic phenomena; to have catostrophic stroke.
I think Dabigratan low dose (110mg bd)therapy will be appropriate in this case to minimise bleeding tendancy.
Its quite interesting that several people have touched upon the use of newer anticoagulants such as dabigatran, rivaroxaban, and apixaban for the treatment of this patient.
The current ‘teaching’ appears to be that if patients on these agents have significant bleeding, it might be difficult to reverse their anticoagulant effect. Therefore, I have seen that patients such as this one are generally anti-coagulated with agents we have more experience with such as heparin (unfractionated or LMW) and warfarin.
It would be great to hear how people feel about this and if they have have had actual experiences with bleeding in patients on novel anticoagulants.
Is current practice based on good data or our lack of experience with newer anticoagulants?
Define and address the mass first and restart low dose Coumadin with goal of inr no greater than 1.5
Interesting patient, with AF and both bleeding and thomboembolism. First the mass has to be further asessed. It may be an LA myxoma as often these are associated with adherent thrombus which may be quite large and embolize (I had such a patient just las week). She may have a coagulopathy or a malignancy. The severe left ventricular hypertrophy in absence of uncontrolled hypertension or aortic stenosis needs to be explored further ?does she have amyloid, hypereosinophilia or is the ‘LVH’ due to left ventricular non-compaction.
Treatment in hospital with iv UFH possibly the ‘safest’ option as can be monitored and quickly reversed if needed; if ‘thrombus’ does not resolve after reasonable length of time then surgical removal is imperative. Any further embolic events make surgery urgent. The newer anticoagulants still pose unacceptable bleeding risk albeit experience is not that extensive.
Excellent case and comments.
This lady as a major bleeding risk (9-12% based on HAS-BLED), and not a lesser stroke risk, wich is 9-10%/yr based on CHA2DS2-Vasc (=7).
So the debate is an option 🙂
I would further investigate this mass (CT as told before).
I would investigate clotting/vascular disorders (p.e. acquired vWF, paraneoplastic syndrome, angiopathy [amyloid p.e.].
I would investigate her GI tract (upper endocopy first) she probably would benefit from PPI.
I would like to know GFR, social support, compliance and cognitive function.
After all this I would debate a further 🙂
Tissue type of LA mass is needed before any changes.
I would first of all suggest an in hospital treatment with LMW heparin or iv UFH, depending on her GFR, to see if the mass resolves. Well, this migth take some time (more than 4 weeks) but as someone said above this seems to be the safest option to be able to react swiftly in case th patient bleeds again. If the thrombus then disappears, I would suggest that a left appendage occlusion, for instance with a watchman device be performed. The patient will then be given clopidogrel for 6 months (ASAP-Study) and ASA as lifelong therapy. I would add a PPI to that regimen to avoid any bad surprise!But if GI-bleeding occurs again during the 6 motns after the above mentioned procedure, since the source of bleeding is known (diverticular), sigmoidectomy will become a serious option.
If the thrombus size remains unchanged under therapeutic heparin concentration, surgical removal will have to be considered.
Repeated bleeding at low therapeutic INR level it make me think about abnormal blood vessels and taking the history of sever headache, SAH, mass in the LA, with multiple thrombosis I would strongly suspect Behcet’s disease .I would recommend doing ESR, ANCA, HLAB27 AND 51, factor 5 LD mutation, ask opinion of rheumatologist. and put the patient on Aspirin and plavix until she has diagnosis.
1. I would use LMW heparin.
2. I would recommend gastroscopy and colonoscopy.
All of the above are excellent recommendation. It is my recommendation that we need a tissue diagnosis of the mass.