September 30th, 2014
No Difference in Survival Found for Different Aortic Valve Prostheses
Larry Husten, PHD
A new study published in JAMA suggests that younger patients who need aortic valve replacement (AVR) may now be more eligible to receive bioprosthetic valves. Each year about 50,000 people in the U.S. undergo AVR surgery. Older patients generally receive bioprosthetic valves because these valves are less prone to clotting and bleeding complications. Surgeons are often reluctant to use bioprosthetic devices in younger patients because these devices are more likely to deteriorate and require a repeat operation.
Researchers at Mt. Sinai Medical Center analyzed data from 4253 patients in New York State who were 50-69 years of age and who received a bioprosthetic or mechanical valve. After adjusting for differences in baseline risk, they compared clinical outcomes associated with the two valves in 1001 matched patient pairs.
There were no differences in mortality or stroke between the two groups. There were more reoperations in the bioprosthesis group, while there were more major bleeding episodes in the mechanical prosthesis group. Here are the cumulative 15-year rates:
- Mortality: 60.6% in the bioprosthesis group versus 62.1% in the mechanical prosthesis group
- Stroke: 7.7% versus 8.6%
- Reoperation: 12.1% versus 6.9%
- Major bleeding: 6.6% versus 13% *
“The absence of a significant survival benefit associated with one prosthesis type over another focuses decision making on lifestyle considerations, including the burden of anticoagulation medication and monitoring, and the relative risks of major morbidity—primarily stroke, reoperation, and major bleeding events,” wrote the authors.
* When originally published, the percentages for major bleeding were accidentally reversed, but have been corrected.
September 29th, 2014
Selections from Richard Lehman’s Literature Review: September 29th
Richard Lehman, BM, BCh, MRCGP
CardioExchange is pleased to reprint this selection from Dr. Richard Lehman’s weekly journal review blog at BMJ.com. Selected summaries are relevant to our audience, but we encourage members to engage with the entire blog.
JAMA 24 September 2014 Vol 312
Prevalence and Incidence Trends for Diagnosed Diabetes Among Adults Aged 20 to 79 Years, United States, 1980-2012 (pg. 1218): A survey of new diagnosis of diabetes in the USA shows a doubling between 1990 and 2008, followed by a plateau, and even, wonder of wonders, a dip thereafter. This is virtually the same plot as obesity over there. And what about over here? I can’t find an up to date plot, although Diabetes UK declares that “Most health experts agree that the UK is facing a huge increase in the number of people with diabetes.” I guess if I looked at the Tesco website a while ago I might have found that “most experts agree that Tesco is facing a huge increase in profits,” but I would have done better to look at the account books.
Lancet 27 September 2014 Vol 384
Losmapimod, a Novel p38 Mitogen-Activated Protein Kinase Inhibitor, in Non-ST-Segment Elevation Myocardial Infarction (pg. 1187): I’ve already warned you that we would be coming upon losmapimod. It is a p38 mitogen activated protein kinase inhibitor. Understand? It doesn’t matter whether you do or not, because losmapimod did nothing at all in this phase 2 trial, where it was given for 12 weeks after non-ST elevation myocardial infarction. At this rate, it won’t even be around long enough to form a useful rhyme word with god.
Ann Intern Med 16 September 2014 Vol 161
Quality-of-Life Outcomes With Coronary Artery Bypass Graft Surgery in Ischemic Left Ventricular Dysfunction (pg. 392): I have been a bit neglectful towards the Annals of Internal Medicine of late, but a sparse week in the other journals led me to look at it. It’s perhaps worth pointing your attention to a randomised trial, which shows that coronary artery bypass surgery can be beneficial for some high risk patients with ischemic left ventricular dysfunction. That was certainly my experience with two youngish patients who were constantly decompensating before they had surgery, but general practice can provide a very misleading sample, and most of my reading since then has suggested that “hibernating myocardium” generally stays asleep, revascularise it as you may. But this trial, on patients with ejection fraction less than 35%, randomly assigned 602 patients to medical therapy alone and 610 to medical therapy plus CABG. The main sustained improvement in the CABG group was in quality of life—very worthwhile, since it can be pretty awful in this patient group.
September 29th, 2014
The Implications of PARADIGM-HF for Clinical Practice
Clyde Yancy, MD and Mary Norine Walsh, MD
CardioExchange’s Harlan M. Krumholz and John Ryan interview Clyde Yancy and Mary Norine Walsh to get their perspectives on the much-discussed PARADIGM-HF trial, previously covered on CardioExchange.
Krumholz and Ryan: Do you see any red flags in this study?
Yancy: These results are among the top five developments in the treatment of heart failure in my 25-year career: ACE inhibitors, beta-blockers, MRAs, device therapy, and now angiotensin–neprilysin inhibition. We have come far, and now with these data the foundation of our treatment regimen has changed yet again. When we change the foundation, it doesn’t mean we’ve raised the bar, it means we’ve elevated the floor. For our discipline and especially for our patients, this is a new day.
I don’t see any red flags in this study. The data are sound, and the design met the top tier of study constructs. However, no study can be comprehensive and no drug or device can be considered the panacea for heart failure, an extraordinarily complex and heterogeneous disease. There are several unanswered questions:
1. The number of trial participants in the U.S. was small, about 5%. Can we be comfortable extrapolating the overall results to the U.S.?
2. The standard of care in PARADIGM-HF involved a lesser degree of device penetration than seen in U.S. heart failure populations: ICD, 15% vs. 30%; CRT, 7% vs. 14%. Would the results remain as relevant in a U.S. treated cohort?
3. Older persons (>75 years) had a favorable point estimate but wide confidence interval. We shouldn’t be compelled by subgroup analyses, but this is a cohort in whom we consistently fail to have adequate data, and it is an ever-increasing patient population in heart failure. We need more data.
4. Patients with NYHA class III and IV heart failure were not well represented in this study. Moreover, study eligibility required tolerability of full doses of either enalapril (10 mg twice daily) or the new regimen, which included valsartan (160 mg twice daily). This may have precluded entry of sicker patients and slanted the study toward mild to moderate heart failure.
5. Perhaps not a red flag but a yellow caution light was that African Americans — the cohort in whom angioedema was most prominent with the drug omapatrilat — were essentially absent from this study. There were about 450 participants of African descent, 219 on active drug. This is a paltry number to reassure us that the risk for angioedema in this group is not elevated. Yet the drug formulation is indeed different, and the overall incidence of angioedema in the study was <1% and not dissimilar between the ACE-inhibitor and angiotensin–neprilysin inhibition groups. We ought be reassured, but given the burden of heart failure in African Americans, we should be certain.
Walsh: Not any red flags per se, but there are some interesting findings in the group studied.
This was a huge study: Approximately 10,000 patients were randomized at 1043 centers. That works out to an average enrollment of 10 patients per site. The relatively low enrollment per site suggests to me that investigators had trouble finding eligible patients, that the patient refusal rate was significant, or both.
The mean blood pressure was 122 mm Hg — pretty substantial when the systolic blood pressure could be as low as 100 mm Hg at enrollment and 95 mm Hg at randomization. This, and the fact that 70% of patients had NYHA class II symptoms at enrollment, suggests that the patients were pretty stable.
A minority of patients were enrolled at sites in the United States, which accounts for the low numbers of patients with ICD and CRT devices: 15% and 7%, respectively.
Each of these factors will come into play when considering this new drug for a patient who is “sicker” with lower blood pressure, poor functional status, and current ICD and/or CRT therapy.
In addition, the sponsor did the initial data analysis. Data transparency is important, and I hope that the sponsor will make the raw data available for analysis.
Krumholz and Ryan: If approved, given what you have seen so far, would you envision switching over many of your patients? And what sort of patients will you use this agent on — for example, patients with a new diagnosis of heart failure? Or will you be stopping ACE inhibition to start this agent?
Yancy: I see this question differently than simply addressing the logistics of “ in whom and when?” I agree that early implementation of this new therapy should be pursued. However, the emphasis is on implementation, which necessarily comes with a new dynamic in clinical medicine — implementation science, or “the study of methods to promote the integration of research findings into practice.” Thus, we should change the paradigm of new drug introduction and proceed diligently with real-time data acquisition, ongoing surveillance for side effects, toxicity, and careful assessment of outcomes, especially hospitalizations for heart failure. Our focus should be on the elderly, those with devices, and African Americans. We ought not just to “make this drug available” but, rather, to create an infrastructure to thoughtfully dispense this therapy in a model that helps us learn as we go.
With that in mind, and with great hope that a sophisticated registry will be place, I would advise following the clinical trial data: Target patients already tolerating full doses of an ACE inhibitor, and proceed with transitioning to the angiotensin–neprilysin inhibitor — or initiating this therapy in newly diagnosed patients while making certain that full doses can be tolerated. Remember, we have data in hand that any dose of an ACE inhibitor improves survival (see the ATLAS trial), but we don’t have the same data for the angiotensin–neprilysin inhibitor.
The rush will be to offer this new drug to the patient failing current therapies. Such use would be empiric, as that is not the scenario in which these data were acquired. We should not offer false hope.
Walsh: The decision to use the new drug, either at the time of a new HF with reduced ejection fraction diagnosis or as a replacement for an ACE inhibitor in a patient with established HFrEF, is a preference-sensitive decision. I see this as an opportunity to share the decision with my patients, after reviewing the results of PARADIGM-HF and any information/restrictions provided by the FDA. My explanation of the trial results and benefits of the new medication will be influenced by my patient’s clinic status and characteristics and how he/she compares with patients randomized in the trial. Ultimately, the decision will rest with the patient.
Krumholz and Ryan: Will cost affect your decision about who should receive this medication?
Yancy: At a population level, no. The only metric that will matter is cost-effectiveness, which certainly should be met given the reduction in hospitalizations. Provided that the cost is not excessive, this therapy should qualify as sound healthcare economics.
But, on a patient level, yes. We see patients already on extensive multidrug regimens and typically with a still-significant out-of-pocket expense for copays. As well, what will happen in community hospitals, especially safety-net hospitals and VA hospitals, where formulary costs are under intense pressure? Unlike for a new iterative therapy, where market forces can predominate in determining cost, for a breakthrough compound that changes the foundation and whose use will be broad, is there a different economic model that might work?
The sign of good research is that more questions are raised and that new opportunities for better care are realized; such is the case here. My hope is that the introduction of this therapy will lessen the burden of heart failure and usher into the clinical sphere a new era in caring for HF patients.
Walsh: Cost will clearly play a role. During the past few years, medical treatment of HFrEF has benefited from the availability of relatively inexpensive, evidence-based generic medications. If approved, how the insurers “tier” this new medication with regard to copayment rules will have a big effect on the out-of-pocket cost to patients. A 90-day supply of 40 mg enalapril tablets is currently available for as little as $10 per month in the U.S. That is a hard price to beat. Each patient will need to consider cost, as well as outcome benefit, and make an informed decision.
September 29th, 2014
American Heart Association: Pay More Attention to Radiation in Imaging Procedures
Larry Husten, PHD
The American Heart Association is urging physicians to better understand the risks of radiation in cardiac imaging procedures. When ordering these procedures physicians should understand the appropriate use of each procedure, the radiation dose associated with the procedure, and the risks associated with that dose. Both the risks and benefits should be fully explained and discussed with patients prior to the imaging procedure.
The full importance of radiation from cardiac procedures is not always appreciated, write the authors of the newly published scientific statement, “Approaches to Enhancing Radiation Safety in Cardiovascular Imaging.” But, according to Reza Fazel, the chair of the writing committee, “heart imaging procedures account for almost 40 percent of the radiation exposure from medical imaging.” The role of radiation is particularly important when considering cardiovascular imaging in younger patients for whom the lifetime risk is likely higher, said Fazel.
The statement urges physicians to discuss several important questions with their patients, including how the procedure will be used to diagnose and treat the patient’s heart problem, whether there are other available techniques that don’t use radiation, how much radiation the patient will receive, and what is known about the risk of cancer associated with the radiation dose.
Fazel offered some overall reassurance: “In general, the radiation-related risk of any imaging test to an individual patient is very small and, when the test is clinically appropriate, the benefits of the test typically far outweigh any potential risks.”
September 25th, 2014
Pulmonary Arterial Hypertension Treatment Worldwide: A Lack of Standardized Care
John Ryan, MD
CardioExchange Editor John Ryan discusses the findings from his new Pulmonary Circulation paper on heterogeneity in pulmonary arterial hypertension (PAH) clinical practice, co-authored with Bradley Maron, MD, of Brigham and Women’s Hospital in Boston and Ghazwan Butrous, MD, of the University of Kent in the U.K.
With the goal of identifying practice patterns, my colleagues and I performed a survey of more than 100 PAH providers worldwide, each with an average of 11 years of clinical experience taking care of patients with PAH.
Among the issues we identified was a lack of standardized care among PAH providers.
We found marked variability in choosing the first line agent with which to treat outpatient PAH (i.e., phospho-diesterase type 5 inhibitors or endothelin-receptor antagonist). Variability is common throughout healthcare, but when the medicines cost >$50,000 per year, we feel that there needs to be more agreement on which agents to choose and how they interact when you go from one medicine to another (currently unstudied). Perhaps of more concern is that, in the setting of cardiogenic shock from PAH, almost a third of providers were not choosing prostacyclins as first-line therapy — even though it is the only proven therapy.
The role of right heart catheterization (RHC) in PAH created controversy. When asked if RHC was a routine strategy to monitor the response to pulmonary vasodilator therapy in PAH patients, the mean score was 4.1 ± 2.0 (Likert scale of 1-7). This suggests considerable disagreement about the role that RHC actually has in following PAH patients. In contrast, there was considerable agreement about the role of echocardiogram in monitoring PAH patients, with a mean score of 6.1 (scale 1-7) — a practice that has not been validated.
The final highlight of our findings is that, when choosing an inotropic agent for these patients, 50% of providers would choose dobutamine as a first line agent — the other 50% would choose one of a variety of agents.
In summary, our survey found that (1) practice patterns among PAH providers vary considerably when it comes to diagnosis, monitoring, and treating patients with PAH and (2) there is no consensus in the treatment of patients with cardiogenic shock secondary to PAH; this is especially alarming because of the upwards of 50-60% mortality associated with cardiogenic shock from PAH.
Are you surprised by the lack of standardized care? What can be done to address this heterogeneity in practice?
September 23rd, 2014
ACC and AHA Release New Guideline for Non–ST-Elevation Acute Coronary Syndromes
Larry Husten, PHD
A new guideline released today by the American College of Cardiology and the American Heart Association reflects important changes in the last 7 years in the understanding of what is now known as non–ST-elevation acute coronary syndromes (NSTE-ACS).
The previous 2007 guideline used different terminology and referred to the same patient population as “unstable angina and non-ST-elevation myocardial infarction (NSTEMI)”. The new terminology “emphasizes the pathophysiologic continuum of unstable angina and NSTEMI and their frequently indistinguishable clinical presentations,” according to a joint press release from the AHA and ACC.
Although NSTE-ACS patients with significant coronary disease are generally eligible for an early invasive strategy, some low-risk patients may be eligible for medical therapy. “Guideline-directed medical therapy has not always been optimally utilized and advances in noninvasive testing have the potential to identify patients with NSTE-ACS at low-intermediate risk to distinguish candidates for invasive versus medical therapy,” says writing committee chair Ezra A. Amsterdam.
The new guideline also drops the term “initial conservative management” in favor of “ischemia-guided strategy” in order “to more clearly convey the physiologic rationale of this approach.”
One major focus is the long-term treatment of patients following the acute phase, said the vice chair of the writing committee, Nanette Wenger. “The hospitalization period involves crisis management of ACS, which is pivotal to successful patient outcomes during the acute phase of disease. However, discharge planning in addition to patient and family education guide the long-term ambulatory care of the patient who has sustained a NSTE-ACS.”
September 22nd, 2014
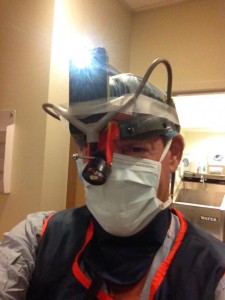
Another MacGyver Moment in Pacemaker Implantation
Shengshou Hu, M.D.
This article was adapted from a post originally published on Dr. Westby Fisher‘s blog.
Installing a permanent pacemaker or defibrillator has become a commonplace event in cardiology these days. During creation of the pocket for the device, I prefer to use a surgical headlamp to visualize bleeding vessels deep within the cavity rather than relying on a conventional overhead surgical light. I have found that headlamps have helped me limit my incidence of post-operative pocket hematoma development. As things have had it, I seem to have a knack for attracting every eighty- or ninety-plus year old who needs an emergency pacemaker on the weekend when I’m on call, and this past weekend was no exception.
So the team was assembled and the pacemaker implantation equipment readied. They knew I liked a headlamp, so they dug deep into the recesses of their inventory to pull out their only headlamp that appeared to be from a bygone surgical era. Being pressed for time, I couldn’t argue and had to make do, but knew that this headlamp might not be very reliable. I saw how the headlamp’s fiber optic cord was secured to the light source that generated about as much light as a few well-lit candles by a cumbersome spring-loaded Rube Goldberg contraption. As I placed the headlamp on my head and tightened the plastic strap, I needed a backup plan in case the light failed.
Would I have to use the overhead light and make do, or might there be another way?
I needed another MacGyver Moment.
That’s when my on-call staff team came up with a brilliant, simplified idea:
iPhone to the rescue!
(PS: This device is experimental and has not been approved by the FDA. Use this device at your own risk. If you experience headaches, nausea, difficulty with concentration, or an erection lasting for longer than four hours, discontinue use of this device and contact your doctor immediately. I have no commercial interest in this device. Also, since the headlamp still worked this weekend, no workaround was needed for the patient, but something tells me we might be getting a new headlamp soon.)
September 22nd, 2014
Selections from Richard Lehman’s Literature Review: September 22nd
Richard Lehman, BM, BCh, MRCGP
CardioExchange is pleased to reprint this selection from Dr. Richard Lehman’s weekly journal review blog at BMJ.com. Selected summaries are relevant to our audience, but we encourage members to engage with the entire blog.
NEJM 18 September 2014 Vol 371
Outcomes One Year After Thrombus Aspiration for Myocardial Infarction (pg. 1111): Sucking clots out of coronary arteries at the time of myocardial infarction sounds like a good idea, and the single centre TAPAS trial, published in 2008, reported a 40% reduction in all cause mortality at one year. Unfortunately it was not designed to measure this outcome, whereas the much bigger Swedish TASTE trial, reported here, was properly powered and better randomized. And would you know it, routine thrombus aspiration before PCI in patients with STEMI did not reduce the rate of death from any cause or the composite of death from any cause, rehospitalization for myocardial infarction, or stent thrombosis at one year.
Follow-up of Blood-Pressure Lowering and Glucose Control in Type 2 Diabetes (OL): The ADVANCE trial was one of the three published in 2007-8, which showed that intensive glucose lowering in established type 2 diabetes had no benefit on hard outcomes. But it was a complex factorial trial, and it also measured the effect of blood pressure lowering in this patient group using a fixed combination of perindopril and indapamide. Once the trial was completed, glucose and blood pressure control in all the groups quickly became similar. So this long term follow-up study is really measuring a “legacy effect” of the tighter control which was imposed many years ago (the median post-trial follow-up period is 5.9 years for blood pressure and 5.4 for glucose). There was no evidence that intensive glucose control during the trial led to long term benefits with respect to mortality or macrovascular events. But the odd thing is that, in contrast with other studies, the tight blood pressure group in this trial does show a small persisting mortality benefit.
JAMA 17 September 2014 Vol 312
Evaluation and Treatment of Older Patients With Hypercholesterolemia (pg. 1136): I’ve just attended the Preventing Overdiagnosis conference in Oxford and I am filled with reforming zeal. So I bridled at the title of this clinical review: Evaluation and Treatment of Older Patients With Hypercholesterolemia. No! and again No! They are people not patients, and there is no such thing as “hypercholesterolemia,” any more than there is such a thing as “hypertension”—there are just continuously distributed variables, which contribute to overall cardiovascular risk. But I was mollified when I read their sensible conclusion: “No RCT evidence exists to guide statin initiation after age 80 years. Decisions to use statins in older individuals are made individually and are not supported by high quality evidence.”
Lancet 20 September 2014 Vol 384
Cardiovascular Outcome Trials of Glucose-Lowering Strategies in Type 2 Diabetes (pg. 1095): It comes as a surprise to me that the Lancet will publish anything with my name on it, but John Yudkin has very kindly sneaked me in as co-author of a letter about cardiovascular outcome trials of glucose lowering strategies in type 2 diabetes. At the time of writing, this correspondence stream seems to have escaped the Elsevier paywall and I would urge you to read it if you can. A response from Rury Holman and colleagues seems to herald a new era of peace and collaboration in finding out the real effects of blood glucose lowering agents. This is exactly what people with diabetes need, and we should seize the opportunity.
The BMJ 20 September 2014 Vol 348
Mild Hypertension in People at Low Risk: While at the conference, I was struck by the number of people who volunteered that they had given up using the term “hypertension” and were keen to see it abolished. I think we should form a Society for the Abolition of Hypertension. Here is an article about mild hypertension in people at low risk that goes some way to explaining why, accompanied by an article by a patient who is fazed by all these seemingly random changes of medical opinion. Take any cardiovascular risk factor, and you see a fairly linear relationship between its level and the degree of risk. Then you put in an intervention that lowers the risk factor. Sometimes it lowers the risk. But in all cases there comes a point where the “disutilities” of the treatment outweigh any benefit, or no benefit is observed at all. At a population level, the treatment may postpone a particular form of mortality, but have no effect on overall mortality. We live a while and then die. We all want to live well, and sometimes have preferences about the way we want to die. So we should all judge for ourselves what treatment we take, based on the likelihood of personal benefit or harm. Nobody suffers from hypertension, but a lot of people suffer from the effects of treatment to lower blood pressure.
September 22nd, 2014
ACC Withdraws ‘Choosing Wisely’ Recommendation Against Revascularization of Nonculprit Lesions
Larry Husten, PHD
The American College of Cardiology said today that it was withdrawing one of its five recommendations in the “Choosing Wisely” campaign. In 2012 the ACC recommended that heart attack patients should have only their culprit artery unblocked. It said that patients and caregivers should question whether complete revascularization of all nonculprit lesions in heart attack patients should be performed.
The original recommendation was based on non-randomized studies suggesting that treating all significantly blocked vessels in heart attack patients could be harmful. “However,” the ACC now states, “over the last two years, new science has emerged showing potential improvements for some patients in their overall outcomes as a result of complete revascularization.”
Two recent randomized controlled trials have altered the field. The 2013 PRAMI trial and last month’s CvLPRIT trial offered evidence that stenting all arteries with large blockages improved the outcome of heart attack patients. But the studies, the ACC states, left many open questions “about the exact timing of the procedures; whether certain patients benefit versus others; whether FFR might guide decisions; and the role of patient complexity and hemodynamic stability.”
“Science is not static but rather constantly evolving,” said ACC President Patrick T. O’Gara, in a press release. He said current clinical guidelines and appropriate use criteria recommendations will also address the impact of the trials. The ACC said it plans to update its “Choosing Wisely” recommendations.
September 18th, 2014
Fellows: Want to Blog for CardioExchange at AHA.14?
Harlan M. Krumholz, MD, SM and John Ryan, MD
CardioExchange editors Harlan Krumholz and John Ryan are looking for fellows to blog for CardioExchange at the American Heart Association Scientific Sessions from November 15th through the 19th in Chicago.
If you are interested in blogging, please contact us. We look forward to hearing from you!